Mohsen Padervand 1, Hadi Salari 1, Faramarz Sadeghzadeh Darabi 2, Mohammad Khodadadi Moghaddam 1, Mohammad Reza Gholami 3
1Department of Science, Islamic Azad University, Ardabil Branch, Ardabil, Iran
2Department of Science, Islamic Azad University, Parsabad Moghan Branch, Parsabad Moghan, Iran
3Department of Chemistry, Sharif University of Technology, Azadi Ave., Tehran, Iran
Correspondence to: Mohammad Reza Gholami , Department of Chemistry, Sharif University of Technology, Azadi Ave., Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
A new approach forward to photocatalytic reduction of nitro compounds was studied, over the clinoptilolate-based silver bromide and silver bromide-titanium dioxide photocatalysts that were prepared and characterized. The photoreaction performed at the presence of NaBH4 which is a strong reducing agent in solution medium. A new mechanism is proposed for nitrobenzene reduction under the photocatalytic conditions. The effect of pH, illumination source, NaBH4 concentration and temperature were also studied. The results showed that the best efficiency obtains over the Ag/AgBr/TiO2/NZ in natural pH under UV light. Also, we observed that the photoreduction efficiency was affected by changing the NaBH4 concentration and temperature. However the effect of former case was more considerable compared to the latter case.
Keywords:
Natural Zeolite, Nitrobenzene, Photoreduction, NaBH4
Cite this paper:
Mohsen Padervand , Hadi Salari , Faramarz Sadeghzadeh Darabi , Mohammad Khodadadi Moghaddam , Mohammad Reza Gholami , "Photocatalytic Reduction of Nitrobenzene with NaBH4 in Aqueous Medium Over the Ag/AgBr/TiO2/NZ and Ag/AgBr/NZ Nanocomposites", International Journal of Materials and Chemistry, Vol. 1 No. 1, 2011, pp. 49-52. doi: 10.5923/j.ijmc.20110101.02.
1. Introduction
Heterogeneous catalysts, and in particular, porous materials such as alumina, silica, and zeolites have many advantages as supports because of their high surface areas, shape/size selectivity and easy separation from reaction mixtures. Mesoporous structures would seem to be ideal for forming a scaffold in which three dimensional dispersions of metal nanoparticles could be supported. Zeolites represent an especially attractive class among the transition-metal-free catalysts for hydrogenation reactions, due to the existence of a large variety of natural/synthetic zeolites having a wide range of acid/base properties[1,2]. Zeolite catalysis and photocatalysis generally includes acid-catalyzed reactions, with many industrial applications in petroleum refining and petrochemical production. However, a number of base-catalyzed zeolite processes have also been reported[3]. Due to the physical properties such as: radiation, mechanical stress, high resistance to heat, low cost, availability and high stability, TiO2 has been greatly considered for the degradation of pollutants and the other chemical process asthe most promising photocatalyst for environmental cleanup [4-6]. TiO2-coated composites practically used thus far have been provided with self cleaning, anti-bacterial and/or anti-fogging functions based on the photo-induced decomposition reaction and photo-induced hydrophilicity[7]. Recently, hydrogenation of nitrates on the surface-modified TiO2-based catalysts has been reported[8]. Titanium dioxide solely or as binary oxides of TiO2/SiO2 has been considered as a potential support for hydrogenation reactions[9,10]. Due to the wide band gap of TiO2 (3.0-3.2 eV), it is active only under near ultraviolet irradiation. Numerous workers have researched methods to modify TiO2 to make it photoactive under visible light[7,11].This work presents the studies of activity of clinoptilolate-based Ag/AgBr and Ag/AgBr/TiO2 nanocomosites. Their activities were evaluated by photocatalytic reduction of nitrobenzene (NB) in aqueous medium.
2. Experimental
2.1. Materials and Methods
Clinoptilolate was used as natural zeolite (NZ) source during this study. Tetraisopropylorthotitanate, cetyl methyl ammonium bromide, nitrobenzene (NB) and all other chemicals were purchased from Merck Chemical Co.Photocatalysts were prepared by sol-gel and deposition method. The precursor solution for preparation of Ag/AgBr /TiO2/NZ contains tetraisopropylorthotitanate, ethanol and nitric acid. The nitric acid was used as catalyst to control the hydrolysis process. Initially, titanium dioxide loaded on the zeolite (25%) by sol-gel method and then, Ag/AgBr particles were deposited on the TiO2/zeolite surface. The preparation procedure for the photocatalysts Ag/AgBr/TiO2/NZ, Ag/ AgBr/NZ and TiO2/NZ has been described in our previous work[12].
2.2. Photocatalytic Test
The photoactivity measurements were done in a quartz reactor that was placed 10 cm in front of a 125 W mercury lamp (OSRAM) and surrounded by a circulating water jacket (Pyrex) to cool the lamp. 0.1 g of photocatalyst was suspended in a 100 mL aqueous solution of 0.01 M (NB). Prior to irradiation, the suspensions were magnetically stirred in dark for 15 min to ensure the establishment of an adsorption/desorption equilibrium among the photocatalyst. At given irradiation time intervals of 20 to 120 min, 1 ml of the suspensions was collected, then centrifuged and filtered to separate the photocatalyst particles.The obtained samples were extracted three times with CH2Cl2, concentrated under a stream of nitrogen and then analyzed by GC/MS. The efficiency of NB conversion could be calculated according to the following formulas: %Photoconversion = C0–C/C0×100Where: C = aniline concentration+ concentration of degradation products= remained concentration of NB C0=initial concentration of NB
3. Results and Discussion
3.1. Influence of Surface Modification on the Adsorption Capability
The adsorption of organic pollutants on the photocatalyst surface (PS) was the important factor that affects the photocatalytic efficiency of heterogeneous photocatalysis. The adsorption capability of Ag/AgBr/TiO2/NZ, TiO2/NZ and Ag/AgBr/NZ was studied and compared with that of pure clinoptilolate are shown in Figure 1.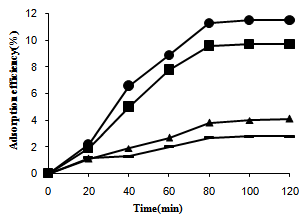 | Figure 1. Adsorption of NB on clinoptilolate (dash), Ag/AgBr/NZ (triangle), TiO2/NZ (square) and Ag/AgBr/TiO2/NZ (circle) |
It was observable that the adsorption capability was greatly enhanced with loading TiO2 on the surface. The adsorption mostly was occurred because of hydrogen bonding between the hydroxyl groups of surface and nitro group of NB.With Ag/AgBr/TiO2/NZ and Ag/AgBr/NZ, furthermore, the columbic interactions could be effective in process of NB adsorption, which was due to the presence of negative and positive species on the surface. 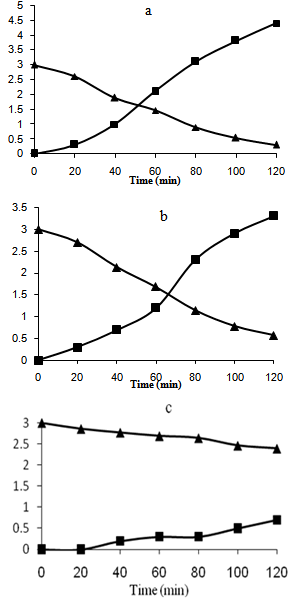 | Figure 2. Photoconversion efficieny under UV light over a) Ag/ AgBr/TiO2/NZ b) TiO2/NZ c) Ag/ AgBr/NZ. (Triangle and Square are 3C/C0(NB) and 103(C-C0)(ainline) versus time, respectively) |
3.2. Photoreduction Mechanism
Figure 2 and Figure 3 shows the results for NB photoconversion and increasing of aniline concentration over the different catalysts in presence of reducing agent (NaBH4) under UV and visible light respectively. The results showed that the best conversion and photoreduction obtained with Ag/AgBr/TiO2 under both UV and visible light. According to the proposed mechanism (equation 1-9), photoreduction of NB to aniline in the medium can be done as a result of two processes. One, reaction between the hydride ion and NB at the presence of H2O in solution, and the other, reduction in the process of producing of electron-hole on the active sites of PS.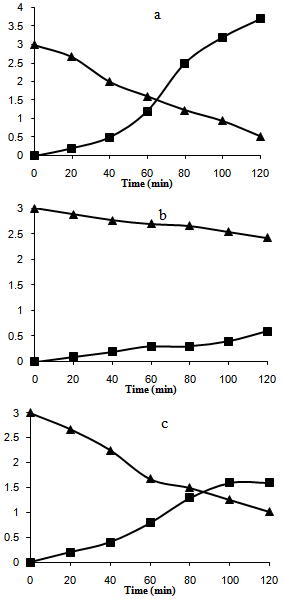 | Figure 3. Photoconversion efficiency under visible light over a) Ag/AgBr/ TiO2/NZ b) TiO2/NZ c) Ag/AgBr/NZ. (Triangle and Square are 3C/C0(NB) and 103(C-C0)(ainline) versus time, respectively) |
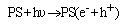 | (1) |
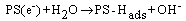 | (2) |
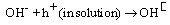 | (3) |
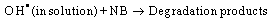 | (4) |
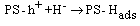 | (6) |
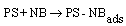 | (7) |
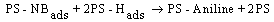 | (8) |
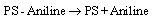 | (9) |
BET analysis showed that Ag/AgBr/TiO2 had highest porosity and surface area after the modification[12]. Under UV illumination, the influence of equations 2 and 6 increases which enhance the efficiency of aniline production according to the mechanism. Under visible light, AgBr is only active species on the PS. The generation of electron-hole is less compared to the former case. It is expected that nanocomposites which is contained silver bromide would have best photoactivity, and the results confirmed this statement. However due to the more hydrophilic and specific surface area, Ag/AgBr/TiO2 photocatalyst is in a higher efficiency.
3.3. Ph Effect
The results showed that the aniline concentration which is the outcome of photocatalytic reduction strongly affect by changing pH. In the basic medium, high concentration of hydroxide anions make important the equations 3 and 4, according to the proposed mechanism. In addition, a competitive treatment appears between the hydroxide and hydride ions throughout the equation 7. Both statements are consistent to enhance the degradation products and diminish the aniline efficiency. On the other hand, in acidic medium a fast reaction between H+ and H¯ inhibits the equations 5 and 6 which are the key steps for generation of aniline. These results present in Figure 4 and show that the best efficiency was obtained when, pH adjusted in neutral amount. 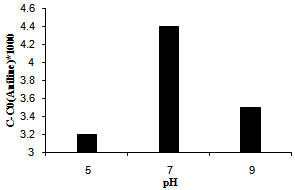 | Figure 4. The effect of pH on photoreduction efficiency over Ag/ AgBr/ TiO2/NZ |
3.4. Nabh4 Concentration Effect
It was observed that an increasing in NaBH4 concentration initially increased the photoreduction efficiency. This observation could be acceptable, by taking a glimpse on equations 5 and 6. However, beyond a limit, photoreduction efficiency does not affected by the high population of H¯ in medium. We suggests that the repulsion columbic interactions of nitro group on the aromatic compound and hydride ions decrease the adsorption of substrate on to PS. In addition, by increasing the NaBH4 concentration beyond of 0.3 M, light penetration into the solution would be less. Thus the generation of electron-hole, which is needed for photoreduction behavior, decreases and the producing of aniline on the PS diminish. The results are shown in Figure 5.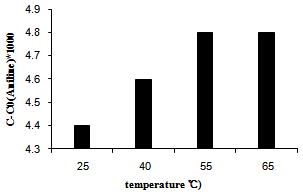 | Figure 5. The effect of temperature on photoreduction efficiency over Ag/ AgBr/TiO2/NZ |
3.5. Temperature Effect
The effect of temperature on the efficiency of NB hydrogenation over the best photocatalyst unde the optimum conditions was investigated and the results presents in Figure 6. Although the overall process of semiconductor photocatalysis is not generally temperature sensitive[13] but, the results indicated that by increasing of temperature, the photocatalytic efficiency increase simultaneously. When the temperature is increased, the collision frequency of species in solution and on the PS increases; this reduces the activation energy, and lead to an increase of reaction rate. However it was observed the photocatalytic efficiency did not show more sensitivity beyond 60˚C. 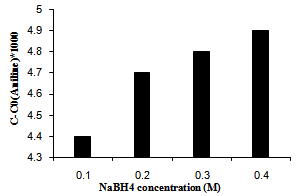 | Figure 6. The effect of reducing agent concentration on photoreduction efficiency over Ag/AgBr/TiO2/NZ |
4. Conclusions
The photocatalytic ability of clinoptilolate-based Ag /AgBr nanocomposites was studied with the purpose of nitrobenzene photoreduction in aqueous medium. The results showed that the best activity was obtained when Ag /AgBr/TiO2/NZ used as photocatalyst under both UV and visible illumination. A mechanism has been proposed for NB photoreduction. The investigation of pH effect on the efficiency of photoreduction reaction indicated that the highest concentration of aniline was achieved in neutral pH which was interpretable in terms of proposed mechanism. According to the results, by increasing the concentration of NaBH4 and increasing of temperature, aniline efficiency enhanced too, while, the influence of former parameter is stronger.
References
| [1] | A. Dyer, Introduction to zeolite molecular sieves, New York, Wiley, 1988, 65 |
| [2] | H. Tsuji, F. Yagi and H. Hattori, Chem. Lett, 1991, 20, 1881 |
| [3] | B. Chan and L. Radom, J. Am. Chem. Soc, 2006, 128, 5322 |
| [4] | Y. Shuhua, J. Yongfeng, S. Zhongliang and Z. Shanlin, J. Photochem and Photobiol., 2010, 86, 1215 |
| [5] | P. Sawunyama, L. Jiang, A. Fujishima and K. Hashimoto, J. Phys. Chem. B, 1997, 101, 11000 |
| [6] | K. Sunada, T. Watanabe, and K. Hashimoto, Environ. Sci. Technol. 2003, 37, 4785 |
| [7] | K. Hashimoto, H. Irie, and A. Fujishima, AAPPS Bulletin, 2007, 17, 6 |
| [8] | N. Krawczyk, I. Witonska, A. Krolak, M. Frajtak and S. Karski, Rev. Roum. Chim., 2011, 56, 595 |
| [9] | P. Reyes, H. Rojas, J. L. G. Fierro, Appl. Catal. A, 2003, 248, 59 |
| [10] | Gao,X.; Wachs, X. E. (1999). Catal. Today. 51, 235 |
| [11] | S. Dezhi, C. Sheng, J. S. Chung, D. Xiaodong and Z. Zhibin, J. Photochem and Photobiol., 2007, 81, 352 |
| [12] | M. Padervand, M. Tasviri and M. R. Gholami, Chem. Papers, 2011, 65, 280 |
| [13] | D. Chen and A. K. Ray, Water Res., 1998, 32, 3223 |




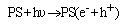
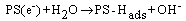
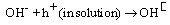
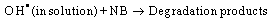
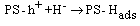
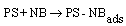
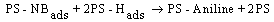
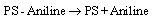



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML