-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2021; 11(1): 1-8
doi:10.5923/j.ijmb.20211101.01
Received: Feb. 4, 2021; Accepted: Mar. 19, 2021; Published: Mar. 28, 2021

Volatile Chemical Constituents and Bioactivity of Selected Piper Species in Sri Lanka
Navodini Jayarathna1, Ruvini Athapattu1, Priyanganie Senanayake1, Priyani Paranagama2
1Department of Plant and Molecular Biology, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka
2Department Chemistry, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka
Correspondence to: Priyanganie Senanayake, Department of Plant and Molecular Biology, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Genus Piper comprises with many economically and medicinally important species in which essential oil is one of the major secondary metabolites responsible for medicinal properties of these plants. The present study was aimed to investigate volatile chemical constituents and in vitro antibacterial, antioxidant and anti-inflammatory activities of essential oils extracted from leaves and fruits of eight Piper species found in Sri Lanka. Plant specimens were collected from natural habitats and cultivations. Essential oils were extracted using steam distillation method and subjected to gas chromatographic analysis. Identification of the volatile chemical constituents was performed by Gas chromatography mass spectrometry (GC/MS) analysis. Antioxidant activity was tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and ferric reducing power assay (FRAP). Human red blood cell (HRBC) membrane stabilizing method was used to evaluate anti-inflammatory activity. To evaluate the antibacterial activity, agar well diffusion assay was conducted for five Piper species and were tested against three pathogenic bacteria: Staphylococcus aureus ATCC 25923, Bacillus subtilis MTCC 121 and Escherichia coli ATCC 25922. As the major volatile constituents of Piper nigrum, β-caryophyllene (60.5-9.1%), caryophyllene oxide (8.49-1.3%), α-copaene (7.4-3.1%), cadina-1,(10)-4-diene (4.3-2.1%), (n)- trans-nerolidol (5.9-0.5%), 4-epi cubedol (11.0-0.5%) and β-linalool (5.7-0.7%) were identified. P. betle was dominated by safrole (39.7-33.0%) and eugenol (43.2-27.4%). Piper longum, P. chuvya and P. sylvestre contained (n)-trans-nerolidol (12.7-0.2%, 66.5% and 41.2% respectively) as the major compounds. P. betle, P. chuvya and P. longum leaves had high antioxidant activity when compared with the standard Butylated hydroxytoluene (BHT). Furthermore, P. betle exhibited high anti-inflammatory activity when compared to the standard (Aspirin). P. nigrum and P. betle showed significant antibacterial activity against S. aureus. Moreover, P. betle exhibited significant activity against B. Subtilis. The bioactivity test results revealed that some of the Piper species available in Sri Lanka are potential sources for developing new herbal drugs.
Keywords: Piper, Essential oil, GC/MS, Antioxidant, Anti-inflammatory, Antibacterial
Cite this paper: Navodini Jayarathna, Ruvini Athapattu, Priyanganie Senanayake, Priyani Paranagama, Volatile Chemical Constituents and Bioactivity of Selected Piper Species in Sri Lanka, International Journal of Modern Botany, Vol. 11 No. 1, 2021, pp. 1-8. doi: 10.5923/j.ijmb.20211101.01.
Article Outline
1. Introduction
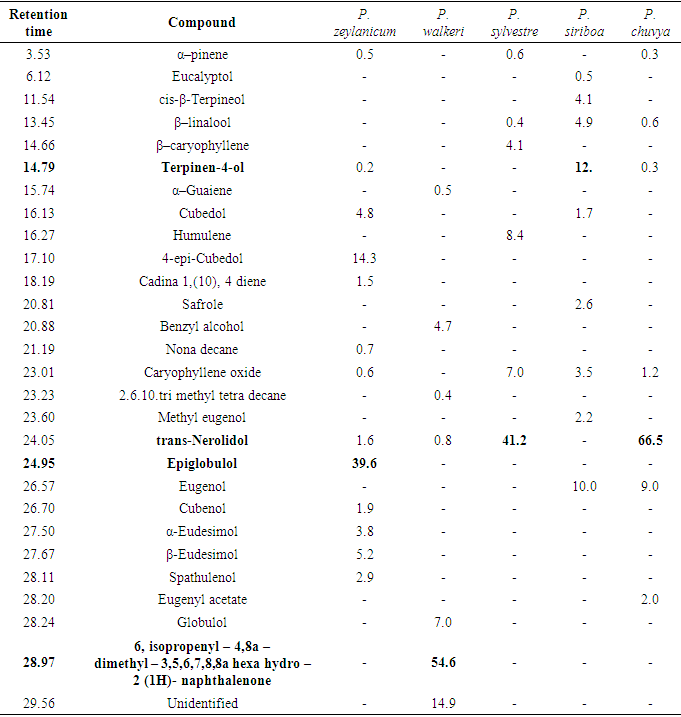
- The genus Piper belongs to the family Piperaceae which includes valuable economically and medicinally important species. Ten Piper species were recorded in Sri Lanka, among them P. nigrum L. and P. betle L. are mainly cultivated and P. longum L. to a lesser extent. Piper zeylanicum Miq., P. trineuron Miq. and P. walkeri Miq. are endemic to Sri Lanka, whereas P. betle, P. longum, P. chuvya, and P. siriboa are considered as introduced. Piper walkeri Miq. is a rare species and P. sylvestre Lam. is the most widespread wild Piper species in the country. Most of the Piper species are used in traditional medicine, which emphasizes the importance of the genus [1-4].Black pepper (P. nigrum), the king of spices is the most widely used spice in the world and its main pungent principle is piperine. The aroma of pepper is determined by the composition of the essential oil usually obtained by steam distillation of black peppercorns [5]. The composition of black pepper oil was reported by several researches. It can be depended on various factors such as harvest year, cultivar, variation in the maturity of raw material, oil extraction method etc. [6]. According to Sruthi et al. [7] variability was observed in aroma quality of black pepper variety Panniyur 1, with respect to altitudes. A clear altitudinal variation was observed in β-caryophyllene and total phenol contents. α- and β-pinene, limonene, myrcene, linalool, α-phellandrene, sabinene, β-caryophyllene and germacrene-D are the main constituents of essential oil of black paper and these compounds contribute for the flavour of pepper [8,9].A study of leaf volatile constituents of ten wild Piper species of Western Ghats using GC/MS, stated that β-caryophyllene, nerolidol and β-elemene as the most abundant compounds in Piper leaf oil [10]. GC/MS analysis of common betel leaf oil indicated safrole (48.69%) and chavibetol acetate (12.55%) as major compounds. It was reported that the composition of the essential oil varied with the maturity [11].Bhuiyan et al [12] has investigated essential oil composition of the inflorescences and leaf of P. longum Linn. grown in Bangladesh by GC/MS. The study revealed the presence of few monoterpene hydrocarbons, a moderate content of sesquiterpenes and high content of aliphatic hydrocarbons. The inflorescences oil was rich in eugenol (33.11%), caryophyllene (9.29%), cinnamyl acetate (5.91%) and β-pinene (4.74%).Sugumaran et al [13] has identified sixty five components in vellaikodi variety of P. betle leaf oil extracted by hydro-distillation method in India. They reported 5-(2-propenyl)-1,3-benzodioxole (25.67%) as the first major constituent in the oil, the second was eugenol, (18.27%) and third, 2-methoxy-4-(2-propenyl) acetate-phenol (8.00%).Bioactivity of all the essential oils revealed both antibacterial and antifungal activities. The effectiveness of the essential oils against different bioactivities varied from each other as chemical composition of the volatiles was different. Terpenes are the main group of compounds present in the essential oils of Piper species which are responsible for their antimicrobial activity [14].Early investigations have been stated that different extractions and essential oils of P. betle, P. nigrum leaves and P. longum fruits contain large number of bioactive compounds including polyphenols, alkaloids, steroids, saponins and tannins hence, possess different bioactivities such as antioxidant, antibacterial, digestive, anti-inflammatory, stimulant, antifungal and nematicidal properties [15-22].Essential oils of black pepper and white pepper showed considerable antioxidant activity against DPPH radicals scavenging activity assay and FRAP assay [22]. P. betle leaves have exhibited activity against Streptococcus pyogens, Staphylococcus aureus, Proteus vulgaris and Escherichia coli. Further, Piperine extracted from P. nigrum fruits and the crude extract has showed the antibacterial activity against S. aureus, Bacillus subtilis, Pseudomonas aeruginosa and E. coli [23].Present study was aimed to evaluate volatile chemical constituents of selected Piper species in Sri Lanka and their antioxidant, anti-inflammatory and antibacterial properties.
2. Material and Methods
2.1. Collection of Plant Material
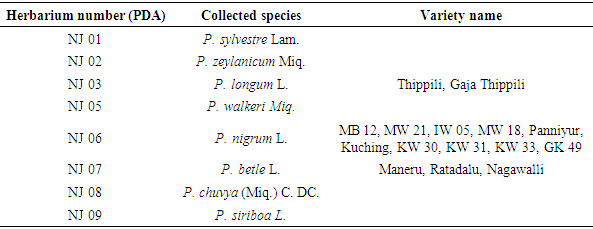
- Healthy plant materials (leaves and fruits) were collected from the cultivations of growers and the field collections of the Intercropping and Betel Research Station (Table 1). Plant specimens were identified by referring to the morphological features provided in “A Revised Handbook to the Flora of Ceylon” [1], by using the authenticated specimens available in the National Herbarium and by using available field collections at the Intercropping and Betel Research Station.Herbarium specimens were prepared and deposited as voucher specimens in National Herbarium, Peradeniya, Sri Lanka (Herbarium numbers are included in the Table 1).
|
2.2. Isolation of Essential Oils
- To collect the essential oil, air dried leaves and fruits (20 g) of Piper spp. (fruits and leaves of P. nigrum and P. longum, and leaves of the other Piper species) were crushed into small pieces and steam distilled by passing steam into the crushed leaves for 3 hours. The condensed solution (400 mL) was saturated with NaCl and subsequently extracted with CH2Cl2 (Analar grade 3, 100 mL) to obtain essential oil. The CH2Cl2 phase was dried with anhydrous Na2SO4 and concentrated using a flash evaporator. A stream of nitrogen gas was passed through the concentrated sample to remove any remaining CH2Cl2 before use in the bioassays and GC analysis.
2.3. Gas Chromatography and Gas Chromatography and Mass Spectrometry
- The essential oils of Piper species (fruit oil of P. nigrum and P. longum and leaf oil of other Piper spp.) were subjected to gas chromatography (GC) to identify the components in the oil. A Shimadzu Gas Chromatograph (GC-2025) with a splitless injector (220°C), flame ionization detector (FID) (270°C), and a fused silica carbowax 20M capillary column (30m 0.25mm) was used under the following conditions: 60°C for 5min followed by 60 to 110°C at 5°C min-1, 110 to 200°C at 3°C min-1 and 200 to 220°C at 5°C min-1 and held for 5 min. Carrier gas was Nitrogen. Gas Chromatography-Mass Spectrometry (GC/MS) was performed using a Thermo Scientific TRACE 1300 Chromatograph fitted with a split-split-less injector, coupled with an ISQ mass spectrophotometer. GC conditions were same as above. The constituents of the volatile oils were identified by comparing retention time with standards.The below mentioned bioassays were tested against leaf oil of P. chuvya, P. sylvestre, P. betle, P. longum and both fruit oil and leaf oil of P. nigrum species.
2.4. Scavenging Ability on 2,2-diphenyl-1-picrylhydrazyl Radicals (DPPH)
- The assay was conducted in a flat bottom 96-well microtiter plate according to the method described by Chatatikun & Chiabchalard [24]. Methanolic DPPH solutions (0.16mM, 40μL) were added to various concentrations of BHT (1.0, 0.25, 0.5, 0.125, 0.0625, 0.03125mg mL-1) in the 96-well plate. All reagents were mixed and incubated for 30 min at room temperature under dark conditions. The absorbance of each well was measured at 517nm with a Microplate Reader (Biotek, USA). Different concentrations of DPPH in methanol solutions were used to plot the standard curve and then the BHT concentration (μg mL-1) in the reaction medium was calculated from the calibration curve. To determine the IC50 (Inhibitory Concentrations), the inhibition rate was calculated and plotted versus test concentrations [25].
2.5. Ferric Reducing Antioxidant Power (FRAP) Assay
- The assay was conducted in a 96-well microtitre plate. Test samples (10 µl), (1.0, 0.25, 0.5, 0.125, 0.0625, 0.03125 mg mL-1), sodium phosphate buffer (0.2 M, pH 6.6, and 25µl) and potassium ferricyanide (1% w/v, 25µl) were added one after the other into wells of the microtitre plate. The similar procedure was performed for the standard (BHT) solution. The control contained all the reagents except the sample. The mixture was incubated at 45°C for 20min, and was terminated by the addition of (10% w/v, 25µl) trichloroacetic acid. This mixture was diluted with 85µl of deionized water and ally freshly prepared ferric chloride (0.1% w/v, 17µl) was added and kept for 10min. The absorbance of the mixture was measured at 700nm, using a microplate reader [26].
2.6. Anti-inflammatory Activity Using HRBC Membrane Stability Assay
- In vitro anti-inflammatory activity of essential oils of different Piper species was assessed by human red blood cell (HRBC) membrane stabilizing method [27] with slight modifications. Fresh blood sample was collected from healthy human volunteers and transferred to heparinised centrifuge tubes. The blood samples were then centrifuged at 3000rpm and washed three times with an equal volume of normal saline. Amount of red blood cells (RBC) were measured and reconstituted as 10% (v/v) suspension with normal saline. The reaction mixture (5.5mL) contained 5mL of the test solution and 0.5mL of 10% RBCs suspension. Saline was added to the control test tube instead of the sample and aspirin was used as the standard drug. All the centrifuge tubes with the reaction mixtures were incubated in a water bath at 56°C for 30min and cooled under running tap water. The reaction mixtures were then centrifuged at 3000rpm for 10 min and the absorbance of the supernatants were measured at 560nm. The assay was performed in triplicate.
2.7. Agar Well Diffusion Method
- Antibacterial activity was evaluated against E. coli ATCC 25922, B. subtilus MTCC 121 and S. aureus ATCC 25923 bacteria as the method described by Jeyaseelan et al. [28]. Bacterial suspension (100μl) was poured on the nutrient agar medium by using a sterile micro pipette and spread evenly. Four wells were developed by using 7 mm diameter sterile cork borer in each Petri dish and two wells were filled with 100μl of tetracycline (0.5mg mL-1) and sterile dimethyl sulfoxide (DMSO) (100μl) as the standard and control. Different concentrations (5mg mL-1, 10mg mL-1 and 25mg mL-1) of isolated essential oils of leaves and fruits of Piper species were prepared. Culture plates were incubated at 37°C for 12 to 18 hours. The zone of inhibition was recorded (in cm) by measuring the diameter of the inhibition zone around the well, including the well diameter. The readings were taken in three different fixed directions and the average values were tabulated. Two replicates were carried out for one concentration of each essential oil. The obtained data were subjected to one way ANOVA (p<0.05) and Turkey’s multiple comparison using MINITAB 17 statistical package.
3. Results and Discussion
3.1. Gas Chromatography Mass Spectroscopy
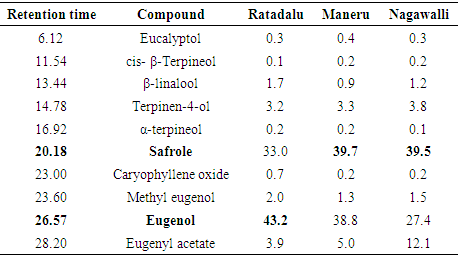
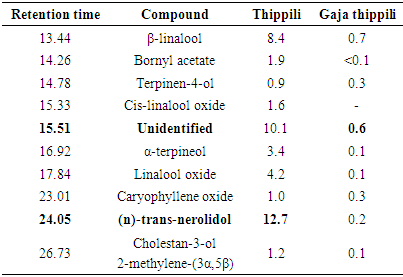
- Major compounds present in essential oils of Piper species are in bold font in each table. β-caryophyllene was the major compound in black pepper varieties and the highest amount was detected in KW33 (60.5%) whereas KW31 showed some alterations by giving an unidentified compound (27.9%) as the major constituent. Figure 1 indicates the gas chromatographs of P. nigrum (MW 18) fruit oil and P. betle (Ratadalu) leaf oil.
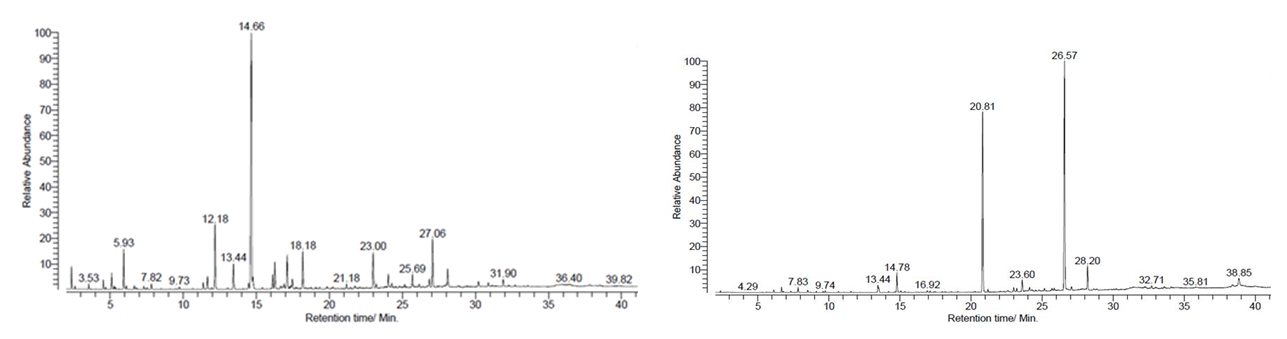 | Figure 1. Gas chromatogram of P. nigrum (MW18) fruit oil and P. betle (Ratadalu) leaf oil |
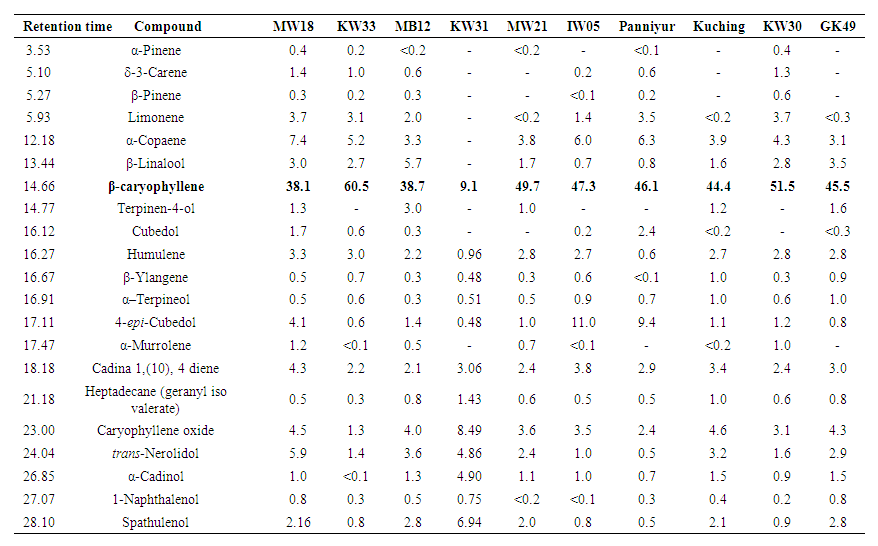 | Table 2. Percentage composition of the essential oils of P. nigrum varieties |
|
|
|
3.2. Scavenging Ability on DPPH Radicals
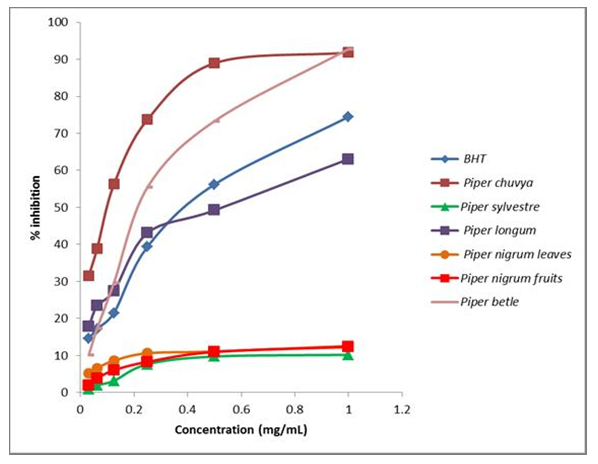
- Total antioxidant activity of the essential oils of different Piper species was determined by DPPH and FRAP assays and compared with the standard (BHT). The positive control, BHT displayed the highest percentage inhibition (74.48%) at 1mg mL-1 concentration and the results are presented in Figure 2.
3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
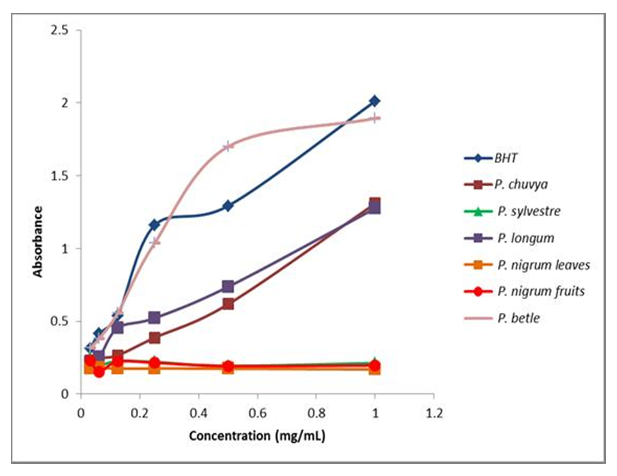
- Activity of the essential oils increased in a concentration dependent manner (Figure 3). Essential oil of P. betle showed remarkable ferric reducing antioxidant power (mean absorbance, 0.982±0.28) comparatively higher than the standard (0.956±0.27). Further, it can be observed that the reducing power increased with the increment of the concentration of essential oil (0.03125-1mg mL-1). These results are in agreement with the findings of Chakraborty and Shah [18].
3.4. Anti-inflammatory Activity
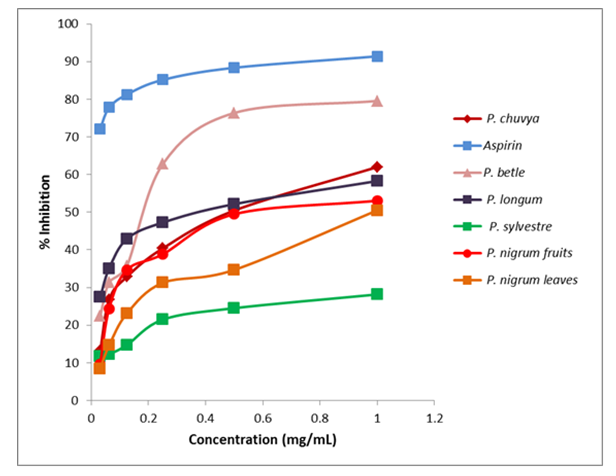
- Total anti-inflammatory activity of the essential oils of Piper spp. was evaluated by the Human Red Blood Cell Membrane (HRBCM) stability assay and compared with the positive control aspirin.Aspirin showed 91% inhibition at the concentration of 1.00mg mL-1 and anti-inflammatory activity of all the essential oils were less than the positive control (Figure 4). Comparatively higher mean inhibition percentage was demonstrated by essential oil of P. betle leaves (51.4%) and it was dose dependent and significantly increased the activity at higher concentration (76% at 0.5mg mL-1 and 79% at 1mg mL-1). It was reported that methanol extracts of P. betle leaves had anti-inflammatory activity by Rintu et al. [22] which is also supported by the present study.
3.5. Antibacterial Assay
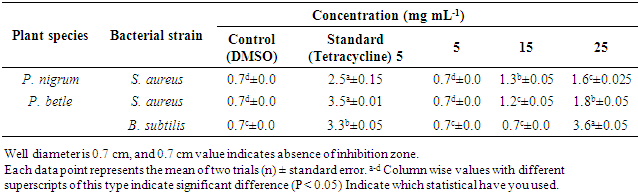
- The present study was focused on evaluating the antibacterial properties of essential oils of five different Piper species by using agar well diffusion assay. The antibacterial assays of the essential oils of P. longum, P. sylvestre, P. chuvya and P. nigrum leaves did not display antibacterial properties at 5mg mL-1 concentration. Inhibition zones were developed against S. aureus when treated with two different concentrations (15 and 25mg mL-1) of essential oils of P. betle leaves and P. nigrum fruit oil separately (Table 6).
|
4. Conclusions
- Major compounds and percentage compositions of volatile chemical constituents in the Piper species were identified. Variations in the percentage compositions of the volatile constituents of varieties belong to the same species can be observed. Essential oils of P. betle, P. chuvya and P. longum leaves had high DPPH free radical scavenging activity compared to the positive control and the high anti-inflammatory properties were observed in essential oil of P. betle leaves. Essential oils of P. nigrum fruits and P. betle leaves showed significant antibacterial activity against S. aureus whereas essential oil of P. betle exhibited significant activity on B. subtilis. The results of this study suggested that some of the Piper spp. available in Sri Lanka are potential sources for developing new herbal drugs.
ACKNOWLEDGEMENTS
- Authors wish to acknowledge Intercropping and Betel Research Station, Narammala, Sri Lanka for allowing sample collection at their site.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML