-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2020; 10(2): 21-26
doi:10.5923/j.ijmb.20201002.01

Physiological Dormancy in Mature Cassava (Manihot esculenta var Brakpo) Seeds
Keania G. Nwako1, Elsie I. Hamadina2
1Department of Agricultural Economics and Extension University of Port Harcourt, Nigeria
2Department of Crop and Soil Science, University of Port Harcourt, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

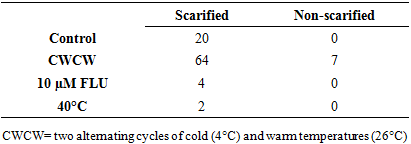
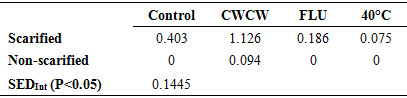
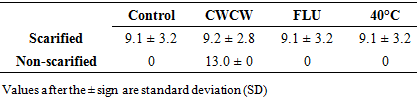
Brakpo is a popular cassava variety in Ogoni land, Nigeria whose mature seeds express low and irregular percentage seed germination. This attribute, which is common in many cassava types, limits its use in seedling production and breeding activities. Although seed dormancy is the well-known cause, the exact form of dormancy involved and its control is unclear. The objective of this study was to determine the effect of seed treatments that regulate embryo growth (two alternating cycles of cold (4°C) and warm (26-30°C) temperatures (CWCW), warm temperature at 40°C and 10 µM Fluridone) and seed coat hardness (scarification and no-scarification) on germination of mature cassava ‘Brakpo’ seeds. The seeds were collected at brown dry fruit stage and subjected to the treatments in a 2x4 factorial experiment arranged as completely randomized design. Treatment included two alternating cycles of cold (4°C) and warm (26-30°C) temperatures (CWCW), warm temperature at 40°C and 10 µM Fluridone) and seed coat hardness (scarification and no-scarification). Scarification alone (control) increased percent germination by 20% compared to zero in the untreated control. Embryo growth treatment were more effective when combined with scarification than without. Among the scarified seeds, embryo treatments increased percent germination in the order CWCW (64%)> FLU (4%)> 40°C (2%). Only CWCW induced germination (7%) in non-scarified seeds. Emergence speed index (ESI) and plumule/radicle lengths followed the same trend. This study suggests that scarification plus two cycles of cold and warm temperature can increase germination to acceptable values. Also, dormancy in Brako may be due more to physiological dormancy (inability of viable embryo to grow) (44%) than hardness of seed coat (20%) or structure of seed coat (7%).
Keywords: Cassava, Dormancy, Germination, Breeding pace, Seed
Cite this paper: Keania G. Nwako, Elsie I. Hamadina, Physiological Dormancy in Mature Cassava (Manihot esculenta var Brakpo) Seeds, International Journal of Modern Botany, Vol. 10 No. 2, 2020, pp. 21-26. doi: 10.5923/j.ijmb.20201002.01.
1. Introduction
- Cassava (Manihot esculenta Crantz) is a member of the family Euphorbiaceae. Globally, it is the fourth most important crop being consumed by about a billion people [1]. In Africa, cassava is a dietary staple and it is highly important in food security because the edible root tuber can store long underground, the plant can thrive under poor environmental and soil conditions and it can grow well under mixed cropping systems [2,3]. The tuber is rich in carbohydrates, calcium, vitamin B and C and essential minerals such as potassium, calcium, zinc, iron, phosphorous. Apart from food, cassava starch is applicable in many types of products such as sweeteners, glues, textiles, paper, biodegradable products, monosodium glutamate, and drugs [4]. Cassava chips and pellets are used in animal feed and alcohol production [4]. The peel and leaves are also used in the production of biofuel and so important as alternative energy source. Cassava yield in Nigeria is lower than the world average (10.6 tons per ha in 2002) and much lower than the yield from many countries cultivating far less area of land to cassava. Barbados for example produces 27.3 tons per hectare in [5]. Thus, the high production values from Nigeria [6,1] are largely due to the cultivation of larger land area than most other countries. To meet the demand for cassava in Nigeria, therefore, productivity must increase well above the world average. A major contributing factor to low cassava yield is the widespread use of low yielding (local) disease susceptible varieties among farmers in Nigeria [7,8]. Profitable commercial cassava production depends on the supply of quality stem cuttings that are high yielding, resistant to prevalent pests and diseases such as cassava mosaic disease (CMD) and unfavorable environmental conditions such as drought, etc., [9]. It is for this reason that cassava breeders constantly breed (through hybridization) to obtain seedlings with superior qualities. The challenge, however, is that the pace at which breeders obtain seed stock for national service cassava multiplication program is slow [10,8]. This has been attributed to the presence of low percentage seed germination [11,7,6] that can be as low as 33% or less when the seeds are collected and planted at fruit maturity stage [6]. Identifying the likely cause(s) of poor seed germination in cassava is key to increasing seed germination. Cassava seeds are commonly collected, in Nigeria, between the months of December and February from stands planted earlier in the year (February-April). These seeds may be stored for up to one year before they are germinated. Viable seeds collected at fruit maturity (i.e., at brown dry fruit stage) are known to stay non-growing for 3 to 6 months while viable seeds that are ready to germination require only about 16 to 40 days to germinate [12]. This observation validates that cassava seed express dormancy.Dormancy is the phenomenon that prevents seeds of many plants from germinating over a long period of time and keeps seeds at different depths of dormancy, which leads to undesirable staggered germination. Primary seed dormancy is caused by conditions either external to the embryo (exogenous dormancy) or within the embryo (endogenous dormancy). While exogenous dormancy is due to: 1.) physical factors of the seed coat that prevent water or gas intake, 2.) mechanical factors such as the hardness of seed coat, 3,) chemical factors such as the presence of chemical inhibitors, or 4.) all the above [13], endogenous dormancy is due to: 1.) physiological factors that relate to the nature of the embryo, 2.) morphological factors of the embryo (underdeveloped or poorly differentiated embryo), or 3.) a combination of both physiological and morphological factors [14]. Some treatments have been shown to improve seed germination. These include scarification, the application of dry heat (at 60°C for 7 or 14 days or 100°C for a few seconds) that mimic bush burning effect and their combinations [15,16,17,8,18]. Also, acid and low temperature stratification have been shown to promote cassava seed germination [9,7]. Scarification and dry heat treatments are thought to act by breaking hard seed coat inhibition, which in turn promotes water/gas uptake and embryo expansion and hence germination while stratification is believed to enhance germination by triggering embryo activity [19] perhaps through the control of abscisic acid [20]. The effect of cold stratification in the absence of scarification is, however, not well known in cassava. Also, although alternating cold (4°C) and warm (26-30°C) cycles are reported to increase the permeability of some seed coats to water and gas and increasing embryo growth, the effects of two and three cold and warm cycles on germination of cassava seeds is hardly reported. Although the role of abscisic acid (ABA) in seed germination is well documented, and ABA is thought to control embryo growth potential, the role of abscisic acid (ABA) and the effect of Fluridone on germination of mature cassava seeds is not widely reported. Fluridone is a herbicide that is also known to inhibit a critical step (i.e., the inhibition of the action of the enzyme required for the conversion of phytoene to the ABA precursor carotenes) in the biosynthesis of abscisic acid (ABA). In view of the above and the fact that cassava seeds contain mature viable embryos even before fruit maturity [21,22], this study proposes that low germination among cassava seeds collected at fruit maturity is controlled by a combination of physical factors of the seed coat and physiological factors of the embryo. Therefore, the objective of this study was to determine the effect of embryo growth regulating treatments (two alternating cold (4°C) and warm (26-30°C) cycles, warm temperature at 40°C and 10 µM Fluridone) and seed coat treatment (scarification or no-scarification) on germination of cassava seeds collected at seed maturity.
2. Materials and Methods
- Experimental locationThe experiment was conducted in the Department of Crop and Soil Science, Faculty of Agriculture University of Port Harcourt, Nigeria to determine to effects of various physical and chemical treatments on germination of cassava seeds collected after fruit maturity. The University of Port Harcourt is located between latitude 04° 31’ to 05° 00N and longitude 006° 45’ 007° 00’E. A propagator was used to store treated seeds under optimum temperature and humidity. The propagator was 2m x 1m x 1m (length, width and height) in dimension with the sides covered with transparent polythene film. Temperature and relative humidity in the propagator were monitored using digital meters.Seed collection and preparationSeeds of the local cassava variety var. Brakpo were collected from a farm in Eleme, Rivers State, Nigeria in January 2015 at brown dry fruit stage, which occurs well after seed and fruit maturity. The collection site is located in humid tropical region, characterized by inherently low fertility soils [23]. Fruit maturity usually occurs between October and December of the preceding year depending on variety. The fruits were wrapped up in a bag, exposed to sunlight and allowed to dehisce. Brakpo was evaluated in this study because it is a popular cassava variety in the area that some farmers in the area described as ‘difficult to germinate’ and so, brought their travail to the attention of the authors. Samples of the seeds were taken for determination of seed weight and moisture content. The seeds were either scarified (by mechanically thinning down the seed coat) or not scarified following the experimental design. Flotation test was then conducted to separate pseudo seed from true seeds and only true seeds were subjected to the experimental treatments. Surface sterilization of good seeds was done using 1.5% sodium hypochlorite solution containing two drops of liquid soup for 5 minutes. All materials used in the study such as forceps, cotton wool etc., were surface sterilized for 10 minutes in 5% sodium hypochlorite solution.Surface sterilized seeds were rinsed in distilled water and then placed in a sterile petri dishes lined with moist cotton wool. Each petri dish was sealed with masking tape to reduce desiccation and then each petri dish was wrapped in a black polythene bag to prevent light and then, they were placed in the propagator. Dark storage is known to promote germination in cassava [17].Experimental TreatmentsTreatments were:1. Non scarified + no treatment (Non scarified control)2. Non scarified + soak in warm water at 40°C for 6 hr3. Non scarified + two alternating cycles of moist cold temperature (4°C) for 2 hr followed by moist warm temperature (26-33°C) for 2 hrs, 4. Non scarified + 10 µM Fluridone for 6 hr5. Scarified + no treatment (control)6. Scarified + soak in warm water at 40°C for 6 hr7. Scarified + two alternating cycles of moist cold temperature (4°C) for 2 hr followed by moist warm temperature (26-33°C) for 2 hrs, (CWCW)8. Scarified + 10 µM Fluridone for 6 hrsTwo alternating cycles of cold and warm temperature treatment This treatment was chosen because alternating freezing and thawing technique is known to improve seed coat permeability to water and gases while moist cold temperature promotes embryo growth potential. However, the effects of alternating freezing and thawing temperatures on germination of cassava seed is not well documented. In this treatment, seeds on moistened cotton wool were exposed to low temperature at 4-5°C for 2 hrs after which the petri dishes were transferred onto a raised concrete floor directly intercepting the sunlight and heat energy. Temperature on the surface of the peri dishes ranged from 26 to 33°C depending on time of day. The cycle was repeated after which, the petri dishes were transferred to the propagator. Experimental design and data collectionThere were two levels of seed thinning treatment and embryo growth enhance treatment at four levels germination inducing treatments. Therefore, the experiment was arranged as a 2 x 4 factorial with 8 treatment combinations, replicated three times and 15 seeds per replicate. Seeds were observed daily, over 30 d from treatment day, for date of radicle and plumule emergence. Data on number of leaves and length of plumule and radicle were recorded.Data analysisPercentage germination Percentage emergence was calculated by dividing the number of germinating seeds per treatment by the total number of treated seeds per treatment and multiplying the result by 100.Emergence Speed Index (ESI)Emergence Speed Index (ESI) was calculated using the equation below:
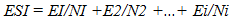 | (1) |
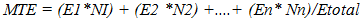 | (2) |
3. Results and Discussion
- Temperature and relative humidity in the propagator and seed parametersAverage temperature in the propagator during the morning, afternoon and evening hours were 20.9°C, 26.5°C and 22.9°C respectively while the relative humidity values were 75, 65% and 86% respectively. Thus, generally, the air temperature in propagator was within favorable ranges for germination. In this study, the average fresh weight of 100 seeds was 8.12 g.Effect of seed treatment on percentage germination In the controls, percentage seed germination moved from zero under non-scarified treatment (experimental control) to 20% when seeds were scarified (treatment control) (Table 1). A zero seed germination in the treatment control shows that seeds of Brakpo cassava cultivar express dormancy as observed by the farmers in its growing area. It also indicates that its dormancy can be slightly managed by using scarification. In this study, the effect of scarification on germination was similar to that reported for other cassava species [7] and seeds of other plant species [27]. The small effect of scarification on percentage seed germination suggests that the dormancy expressed by Brakpo is due only to a small extent by the presence of hard seed coat. Dormancy caused by the presence of hard seed coat has also been reported in over 15 angiosperm families [14,12] but not in the cassava cultivar Brakpo.
|
|
|
|
4. Conclusions
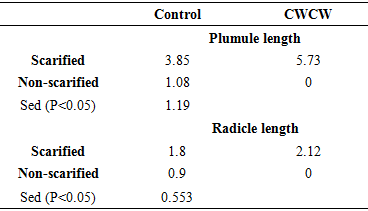
- This study has shown that scarification (thinning down of seed coat) needs to be combine with treatments that enhance embryo growth to obtain high germination rate in cassava (Brakpo). The combination of scarification and two cycles of cold (4°C) and warm temperature (26-30°C) for 6 hrs each time can increase radicle and plumule germination of mature cassava seeds to as high as 64%. This combined treatment also increased radicle emergence speed index (ESI) and enhanced early seeding growth (radicle length). Also, it showed that warm temperature alone was not enough to stimulate embryo growth in scarified seeds, and ABA may play a role in the control of embryo growth. This imply that dormancy in Brako is determined by physiological dormancy (inability of viable embryo to grow) (44%), followed by hardness of seed coat (20%) and structure of seed coat (7%). The findings from this study could help improve the selection process in cassava breeding. Further studies that compared CWCW and cold temperature treatments may be investigated as well as studies that test the effect of a combination of cold temperature and FLU, FLU alone and ABA alone.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML