-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2020; 10(1): 15-20
doi:10.5923/j.ijmb.20201001.03

Scarification and Low Temperature Stratification Effects on Germination of Mature Seeds of Cassava (Manihot esculenta) Hybrids
Boma B. George1, Elsie I. Hamadina2
1Department of Agricultural Economics and Extension University of Port Harcourt, Nigeria
2Department of Crop and Soil Science, University of Port Harcourt, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The cause of low germination percentage among hybrid cassava seeds of TMS 98/0505 and NR 87184 is not well elucidated and the effect of seed treatments such as scarification and low temperature stratification (4°C) on seed germination and early seedling development of TMS 98/0505 and NR 87184 is not well known. Thus, the objective of this study was to determine the effect of non-scarification (control), scarification alone and scarification + low temperature stratification (4°C) on germination percentage and early seedling development of two cassava hybrids (TMS 98/0505 and NR 87184). The seeds were collected 7 months after harvest at brown dry-fruit stage and given one of the three treatments. Results showed that the combination of scarification and low temperature (4°C) stratification increased radicle germination by 10% in TMS 98/0505 and 7% in NR 87184 while germination was only 3% in the control. In both hybrids, plumule emergence was at least doubled by the application of scarification and low temperature stratification compared to the control. Emergence speed index (ESI), and radicle length were fastest and longest respectively in the low temperature stratification treatment (particularly in TMS 98/0505) and slowest/shortest in the control. This study has shown that the application of low temperature stratification on scarified seeds increases germination rate of dormant cassava hybrids. It also suggests that the low seed germination percentage observed may be caused by dormancy due to a combination of seed coat and embryo (physiological) factors. Every increase in germination would promote propagation through seed as well as enhance rate of cassava improve through convention breeding. Potentially more effective method of scarifying and stratifying these seeds for greater impact are suggested herein.
Keywords: Cassava, Scarification, Stratification, Germination
Cite this paper: Boma B. George, Elsie I. Hamadina, Scarification and Low Temperature Stratification Effects on Germination of Mature Seeds of Cassava (Manihot esculenta) Hybrids, International Journal of Modern Botany, Vol. 10 No. 1, 2020, pp. 15-20. doi: 10.5923/j.ijmb.20201001.03.
Article Outline
1. Introduction
- Low seed germination (<33%) and uneven germination (15- 80%) of true cassava (Manihot esculenta Crantz) seeds are major constraints to breeding for high cassava yield, contributes to the widespread use of low yielding, disease susceptible varieties and dwindling cassava seed production in Nigeria [1,2,3]. Commercial cassava production depends highly on the supply of quality stem cuttings (planting material) that are high yielding, resistant to prevalent pests and diseases such as Cassava Mosaic Disease (CMD) and unfavorable environmental conditions such as drought, etc. [4]. Such quality stem cuttings are products of breeding (hybridization), whose production depends highly on the availability of large quantity/quality of germinable seedstock and hence large number of vigorous seedlings/plants with diverse traits to select from in breeding programs [4,5]. Cross breeding of plants with desirable traits encourage the recombination of genes that increase the chance of obtaining seeds with high resistance to pest and diseases, drought etc. Low seed germination also makes seed collection unattractive, which in turn affects the conse rvation of cassava genotypes in seed form. This downplays the importance of cassava seeds as a better means of storage than the stems. Cassava seeds are known to have the capacity to stay viable for as long as one year while stem cuttings remain viable for only about three weeks. Moreover, the transportation of seeds as propagule is much easier [6], and seeds generated from natural hybridization have combined genes suitable for higher productivities, resistance and adaptability than clones [7].Dormancy is the one physiological phenomenon that is known to regulate the timing of germination in seeds or plant parts that express low and delayed germination. In cassava, such seeds are dormant for 3-6 months while their non-dormant counterparts require about 16 – 40 days to germinate [8[. Even among the cassava seeds approaching their natural germination time, the problem of low and staggered germination is still commonly observed. Thus, continuous studies to solve this problem is highly necessary because cassava (a member of the family Euphorbiaceae, is the fourth most important food crop in the world. Also, it is consumed by about a billion people [9] globally, and in Africa, it is a dietary staple important in food security [10,11]. Identifying the likely cause(s) of poor seed germination in cassava is key to increasing seed germination. Primary seed dormancy prevents seeds from germinating and leads to staggered germination at the end of dormancy. It is also clear that dormancy may be caused by conditions either external to the embryo (exogenous dormancy), within the embryo (endogenous dormancy). Exogenous dormancy could be due to: 1.) physical factors of the seed coat that prevent water or gas intake, 2.) mechanical factors such as the presences of hard of seed coat that make it difficult for the embryo to emerge, or 3.) Chemical factors such as the presence of chemical inhibitors [12]. Endogenous dormancy, on the other hand, may be due to: (i) Physiological factors that relate to the nature of the embryo, (ii) Morphological factors of the embryo (underdeveloped or poorly differentiated embryo) or (iii) a combination of both physiological and morphological factors [13]. Unfortunately, the cause of dormancy in cassava is not clear [2], and how these or their combination affect cassava seed germination is not so clear.Efforts to increase percentage germination in cassava are few. Based on the reported durations from treatment to germination in control treatments [14,15,16,17,18], it appears that many of the studies were conducted on seeds that had been stored up for some months prior to the study and so close to their natural germination time. In seeds of this age, scarification (mechanical or chemical), and dry heat (at 60°C for 7 or 14 days or 90°C for a 2 mins) have been shown to lead to 16- 79% germination as against 80% germination in the control. These seed treatments are thought to act by breaking the hard seed coat inhibition, which in turn promotes water/gas uptake and embryo expansion and hence germination. Thus, seed coat related factors (referred to as exogenous dormancy, i.e., dormancy caused by factors external to the embryo such as hardness of seed coat and/or presence of impermeable seed coat to water and gases), are known to be a strong factor determining germination rate at this seed age. Another promising method identified by is stratification. Although warm temperature stratification has not significantly increased percentage germination in some cassava seeds [18,20], its ability to promote germination has been reported [12,13]. On the other hand, the effect of low temperature stratification on cassava seed germination has not been widely reported. The report of [21] suggested that temperatures of 0-7°C cures physiological dormancy in seeds, but its effect on hybrid cassava seeds have not been reported. The need to investigate the effect of cold temperature stratification is further driven by the insight from the work of [20] who showed that two alternating cold and warm temperature cycles significantly increased germination percent (>60%) in scarified seeds of a highly dormant local cassava variety var ‘Brako’. Because stratification enhances germination by triggering embryo activity [21] or increasing seed coat permeability further strengthens the need to investigate the effect of cold stratification on germination of mature seeds dormant hybrid cassava seeds. Lastly, since cassava seeds are commonly collected (in Nigeria) between the months of December and February from stands planted between February-April and are sometimes stored for up to one year, it is necessary to determine how scarification (a seed coat thinning treatment) and cold stratification would affect germination of hybrid cassava seed stored up for months after harvest. Therefore, the objective of this study was to determine the effect of seed coat treatment (non-scarification, scarification and scarification + low temperature stratification; 4°C) on percentage germination of seeds of two hybrid cassava seeds that have been stored up for one year.
2. Materials and Methods
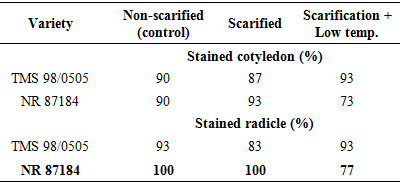
- Experimental locationThis study was conducted in the Department of Crop and Soil Science, Faculty of Agriculture University of Port Harcourt, Nigeria (04° 31’ to 05° 00 N and longitude 006° 45’ 007° 00’ E). Treated seeds were placed on moist cotton wool lined petri dishes, which were in turn placed in a propagator. The propagator was 2m x 1m x 1m long, wide and high with the sides covered with transparent polythene film. Temperature and relative humidity in the propagator were monitored using a digital sensor.Seed collection and preparationSeeds of two hybrid varieties of cassava Manihot escenlenta; cultivars TMS 98/0505 and NR 87184 were collected in the month of August 2015 from National Root Crop Research Institute (NRCRI), Umudike, Nigeria, located between latitude 05° 28’ 33’’ N to 007° 32’ 56’’ E and longitude 005° 48’ N to 007° 55’ E, with average rainfall of 2000-2500mm. The seeds were harvested in January 2015 after fruit maturity (at brown dry fruit stage) and stored up in brown paper bags, under room condition in NRCRI until August 2015. These varieties were chosen because they hybrids whose seeds are known to exhibit long seed dormancy and low percentage germination but are high yielding and resistant to cassava mosaic disease as well as many major pests of cassava [2,19]. Indeed, the two varieties are part of the twelve hybrid cassava varieties released for adoption to farmers in 2014. Ten groups of 100 dry seeds were randomly selected from each variety and weighed. Out of the 1000 seeds weighed, about 200 were left un-scarified while the rest were scarified. Scarification was done manually to take off a small patch of the seed coat close to the caruncle. Flotation test was done to separate pseudo seeds from filled seeds and only those that sunk were included in the study. Surface sterilization of true seeds was done using 1.5% sodium hypochlorite solution containing two drops of liquid soup for 2 minutes, then they were rinsed in distilled water and placed in a sterile petri dishes lined with moist cotton wool. There were 50 seeds per petri dish, replicated three times. Each petri dish was a replicate. All petri dishes were sealed with masking tape to reduce desiccation and then each petri dish was wrapped in a black poly bag to prevent light and then, they were placed in the propagation. Dark storage promotes germination in cassava [16]. All materials such as forceps, cotton wool etc., were surface sterilized for 10 minutes in 5% sodium hypochlorite solution.Experimental TreatmentsThere were two factors: cultivar (TMS 98/0505 and TMS 98/0505) and seed treatment (non-scarification (control), scarification alone and scarification + low temperature stratification (4°C). Thus, there were six treatment combinations in which all the seeds were scarified before treatment application. In the experimental control (i.e., non-scarification, the seeds were not scarified. Low temperature stratification was not tested on non-scarified seeds because the preview of a previous study [20,18] using local variety showed only slight effect of warm stratification on germination of non-scarified seeds. Non-scarified (experimental control) control set of seeds were however included in other to determine the length of dormancy of the seed lot. Low temperature (0-4°C) stratificationThis treatment was chosen because cold stratification is known to be a cure of physiological dormancy (dormancy due to low embryo growth potential) and physical dormancy (by inducing membrane permeability). To achieve this, fifty scarified seeds were placed in each of three petri dishes lined with moistened cotton wool and exposed to low temperature at 4-5°C for 2hrs. Thereafter, the petri dishes were taken out, wrapped up in black polythene bags and place in the propagator.Seed viability test using tetrazolium chloride solutionSeed viability test using 0.5% tetrazolium chloride solution was conducted at the end of the study. Ten un-germinated seeds were collected from each petri dish. The seeds were preconditioned by soaking them in water for 24 hours at a temperature of 30-35°C. The seeds were dissected longitudinally through the embryo. One half of each seed was placed in 0.5% tetrazolium chloride solution for 6 hours. The seeds were examined for stained radicle and cotyledon. This was done to determine whether the un-germinated seeds did not grow due to loss of viability perhaps imposed by the experimental treatments.Experimental design and data collectionThe experiment was a 2 x 3 factorial with six treatment combinations. The experiment was arranged as a completely Randomized Design with three replications. The seeds were observed daily for date of radicle and plumule emergence. At the end of the experiment (i.e., at 52 days after treatment), data was taken on number of leaves, radicle length and leaf length and width. Two seedlings per treatment combination per replicate were sampled for this assessment.Data analysisEmergence Percentage (EP)Percentage emergence was calculated using the equation below:
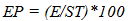 | (1) |
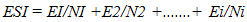 | (2) |
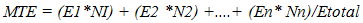 | (3) |
3. Results and Discussions
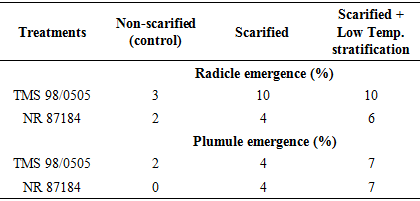
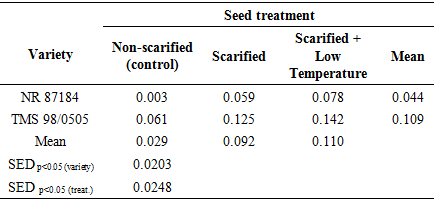
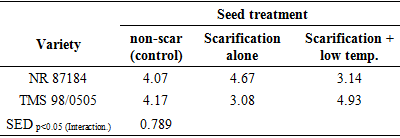
- Temperature and relative humidity in the propagatorAverage temperature in the propagator during the morning, afternoon and evening hours were 20.9°C, 26.5°C and 22.9°C respectively. Thus, generally, the air temperature in propagator was within favorable ranges for germination. Seed weightThe average seed weight per 100 seeds was 13.03 g ± 3.074 (SD) and 9.59 g ± 0.287 (SD) in NR 87148 and TMS 98/0505 respectively. Thus, NR 87148 had heavier seeds than TMS 98/0505. The seeds of NR 87148 also appeared larger than those of TMS 98/0505.Effect of seed treatment on percentage germination of two varieties of cassavaPercent germination was generally higher in TMS 98/0505 than NR 87184 (Table 1). Compared to the control (non-scarified), scarification and scarification + 4°C temperature treatments increased percentage radicle emergence by about than 3x in TMS 98/0505 while they (at least) doubled the percentage germination in NR 87184. Therefore, TMS 98/0505 had better tendency to germinate as well as had more positive response to scarification and its combination with low temp. treatments than NR 87184. In both varieties, plumule emergence was at least doubled by the application of scarification or scarification + 4°C temperature treatments.
|
|
|
|
4. Conclusions
- In conclusion therefore, this study has shown that hybrid seeds of TMS 98/0505 and NR 87184 cassava variety exhibit very rate of germination which implies that breeders working with these varieties would normally find it difficult to obtain high seedling population to screen from in the search for desirable traits. This study has also shown that TMS 98/0505 shows higher tendency to germinate than NR 87184 whether in nature or under treatments that purported to enhance germination. Although scarification is known to promote seed germination in many seeds as well as in the varieties examined here, the combination of scarification and low temperature (4°C) further increased percentage radicle and plumule emergence. This study therefore suggests that the low germination rate of seeds of TMS 98/0505 and NR 87184 is not caused by the presence of hard seed coat alone but even more by the presence of physiological dormancy (an inherent inability of viable embryos to grow). While scarification alleviates issues of hard seed coat/ difficulty of seeds to take up water and gas, the combination of scarification and low temperature (low temperature stratification) treatments stimulates embryo growth. Although the results from this study were impressive compared to the control, the 10% germination achieved in this study calls for more studies to further increase percentage germination in these varieties. Further studies may consider scarification of the entire seed surface combined with low temperature treatment or a combination of scarification of the entire seed surface and two alternating cycles of low and warm temperatures.
ACKNOWLEDGEMENTS
- The authors wish to thank Dr. Chiedozie Egesi, of National Root Crop Research Institute Umudike, Nigeria, for providing the cassava hybrids used in this trial.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML