-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2018; 8(1): 15-23
doi:10.5923/j.ijmb.20180801.03

Thidiazuron Induced Differential Regenerative Response in Embryo Axis Explants of Milletia pinnata (L.) Panigrahi
Sujatha Raman1, Sulekha Hazra2
1Dept. of Environmental Sciences, Savitribai Phule Pune University, Pune, India
2National Chemical Laboratory, Pune, India
Correspondence to: Sujatha Raman, Dept. of Environmental Sciences, Savitribai Phule Pune University, Pune, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Thiadiazuron (TDZ), induces morphogenesis almost in any plant tissue depending on species. In Pongamia/ Milletia, TDZ is reported to trigger normal adventitious organogenic pathway in the de-embryonated cotyledonary segments. But it failed to trigger normal caulognic buds in embryo axis explants. The present study was conducted to understand the role of TDZ in the mechanism of induction of de novo caulogenic buds and failure of differentiation of these buds at the tissue level using histology. Also, a comparative histological study was undertaken with de-embryonated cotyledon explants that produced normal caulogenic buds that got differentiated into shoots. In both explants, the meristematic activity was triggered in the subepidermal layers and wounding triggered more morphogenic response. In de-embryonated cotyledon explants or control, a normal morphogenic pathway was induced by TDZ that lead to normal shoot primordial differentiation. However, we demonstrate that in embryo axis explants, anomalous meristematic activity proceeded toward teratological protuberances that resulted in lack of shoot primordial development. This could be due to altered TDZ signaling in embryo axis explants without cotyledon attachment. This drives attention towards the critical factors regulating TDZ signaling.
Keywords: Thidiazuron, Embryo axis, Organogenesis, Histology, Cotyledon
Cite this paper: Sujatha Raman, Sulekha Hazra, Thidiazuron Induced Differential Regenerative Response in Embryo Axis Explants of Milletia pinnata (L.) Panigrahi, International Journal of Modern Botany, Vol. 8 No. 1, 2018, pp. 15-23. doi: 10.5923/j.ijmb.20180801.03.
Article Outline
1. Introduction
- Among the GRs, TDZ has shown to have both auxin and cytokinin like effects, although, chemically, is a phenylurea derivative, unlike aminopurines. A number of biological events in cells are induced or enhanced by TDZ [6, 11, 13, 18, 22, 24] it has been proved to have a potential in elucidating de novo organogenic response in various species like Saussurea involucrate [10], blueberry and blackberries [4], Adhatoda casica [16], Pelargonium capituatum [3], Nothapodhytes foetida [30], Lens culinaris [12], Tamarindus indica [17, 18], etc.Guo et al. [9] reviewed the multi-dimensional role of Thiadiazuron (TDZ), that has a wide array of physiological responses in different plant species, different explants etc. Though TDZ induces varied biological events in the cells, its mode of action is yet clearly unknown. Most of the reports emphasized the concentration of TDZ to be used in culture to get the desired response. The response of TDZ varied with the explants tested in wide varieties of species. It is well documented in the literature that the cotyledonary explants with intact embryo axis respond better in culture [21]. Similarly, embryo axis with attached cotyledon respond well [1]. Shoot bud regeneration is promoted by all GRs, including TDZ in embryo axis explants of many species. To the best of our knowledge, direct adventitious organogenesis induced by TDZ alone, resulting in normal plantlets in embryo axis explants without cotyledon attachment is not yet reported among any plant species. In Milletia pinnata (L.) Panigrahi, often known by the synonym Pongamia pinnata, clonal propagation of this species from juvenile [27] and mature tree derived existing meristems [28] has been reported. Role of TDZ in inducing adventitious organogenesis in Pongamia/ Milletia was studied in embryo axis and de-embryonated cotyledon explants. Among the explants studied, the de-embryonated cotyledon segments responded well in terms of caulogenic bud induction and its differentiation into the shoot [29]. Though embryo axis explants have also produced caulogenic buds, they were not differentiated into the shoot.
2. Objectives
- The present study was conducted to understand the role of TDZ in the mechanism of induction of de novo caulogenic buds and failure of differentiation of these buds in the embryo axis explants at the tissue level. Efforts were taken to understand the mode of induction by TDZ using histology and a comparative histological study was undertaken with de-embryonated cotyledon explants that produced normal caulogenic buds that got differentiated into shoots [29].
3. Materials and Methods
3.1. Culturing of Embryo Axis
- Nearly mature, hard, green pods were collected from the Pongamia/ Milletia trees grown locally. The pods were washed in running tap water and were treated with 1% Bavistin (Carbendezim 50%WP, BASF, India) for an hour containing few drops of detergent (labolene, India) on a gyratory shaker at a speed at 90 rpm. After this treatment, the pods were washed with sterile distilled water 3 to 4 times under sterile conditions. This was followed by a treatment with 4% Savlon (Johnson and Johnson, USA) for 10 min. Pods were washed again with sterile distilled water twice. Finally, the pods were treated with 0.1% mercuric chloride for 10min. To eliminate adhering HgCl2, the seeds were rinsed repeatedly for four times with sterile distilled water under aseptic conditions.Surface sterilized Pods were cut opened aseptically and the seeds were isolated. Seed coat was excised. Cotyledons were separated into two such that the embryo axis can be excised without any damage.The excised embryo axis was cultured on to Murashige and Skoog’s (MS) basal medium [20] with 2% sucrose supplemented with different concentrations of TDZ (0.45, 2.27, 4.54, 6.81, 9.08, 11.4, 13.6 & 22.7μM) in 90mm petridishes. MS basal medium without TDZ was used for control. The embryo axis explants were cultured in TDZ supplemented MS media for two different incubation period viz. 10 and 20 days with 12 replicates per treatment and per incubation. After respective incubation period, the explants were transferred to GR free MS basal media for four passages of 15 days each. Data on the frequency of response and numbers of shoot buds per explants were noted after four passages of 15 days each in GR free MS basal media. The experiment was repeated five times with 12 replicates per treatment per incubation for reproducibility.Thereafter, only the explants showing meristematic buds were cultured onto MS basal medium supplemented with 0.45μM TDZ, or 4.45μM BA, or 0.45μM GA3 for bud differentiation.In all experiments, the pH of the MS media used was adjusted to 5.8 after supplementation of TDZ. The media were gelled using 0.7% agar (Hi Media, India) and were autoclaved at 1.06Kgcm-2 for 20 min at 121°C. All the cultures were incubated at the cool white light at an irradiance of 16µ Em-2s-1 with 24 h photoperiod at 25 ± 2 °C. Completely Randomized designs were used. T he data were subjected to statistical analysis (ANOVA) and treatment means were compared [23]. Data was analyzed using Microsoft Excel package.
3.2. Histological Studies
- Sections were prepared for histological studies following the methods described by Sharma and Sharma [26]. Histological studies were carried out in embryo axis tissues cultured initially in different concentrations of TDZ (4.54. 11.35 & 13.6μM) for 20 days and followed by 30 days in GR-free MS media. These explants were cut into small (approx 3 x 4 mm) pieces and were fixed in FAA (formaldehyde: glacial acetic acid: alcohol, 5:5:90, v/v) for 48 h at room temperature. Tissues were dehydrated using graded concentrations of tertiary butyl alcohol and embedded in paraffin wax (mp 58-60°C). Serial sections of 10 μM were cut using a rotary microtome (Reichert-Jung 2050, Germany). Sections were double stained with hematoxylin-eosin and mounted with DPX (Loba Chemie, Mumbai, India) for studies under a microscope attached with b/w CCD camera. For a comparative study, tissues from proximal segments of the de-embryonated cotyledon [29] explants, incubated with different concentrations of TDZ (0.45 μM, 11.35 μM & 13.6μM) supplemented MS basal media for 20 days and followed by 10 days and 30 days in GR free MS media were subjected to histological examination. The tissues segments were cut into small pieces. The tissues were fixed, dehydrated, stained and sectioned following the methodology mentioned for embryo axis tissue sections.
4. Results
4.1. De Novo Direct Response of Embryo Axis in TDZ Media
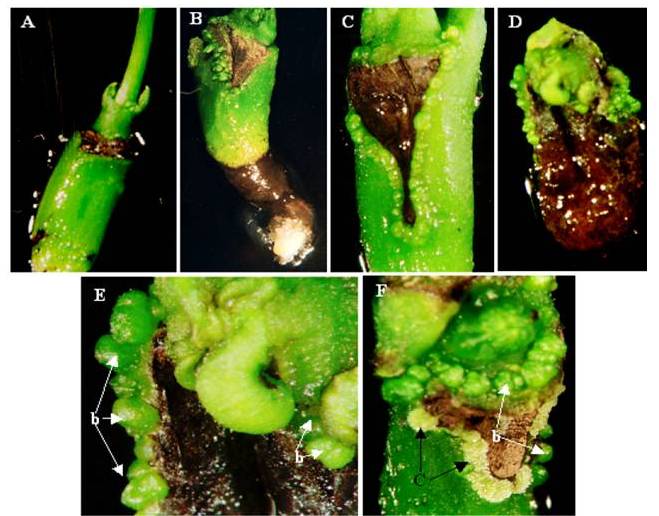
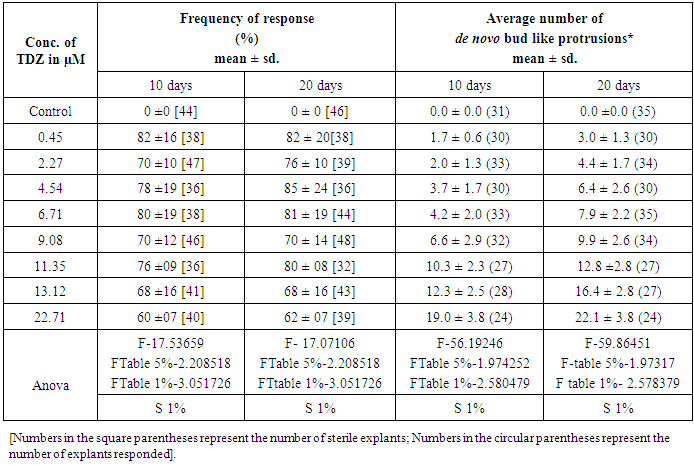
- The embryo axis explants germinated normally with the frequency of 70 % in TDZ free MS basal media, and no adventitious bud formation was observed (Figure 1A). Whereas, bud-like structures were induced in all cultures exposed to TDZ for 10 days and 20 days (Table 1). The frequency of response in producing adventitious bud-like structures ranged from 60% in 22.71μM to 82 % in 0.45μM TDZ in 10 days exposed explants. In 20 days TDZ preconditioned explants, the frequency of de novo caulogenic response ranged from 62% in 22.71μM to 85% in 0.45μM TDZ.
|
4.2. Histological Studies
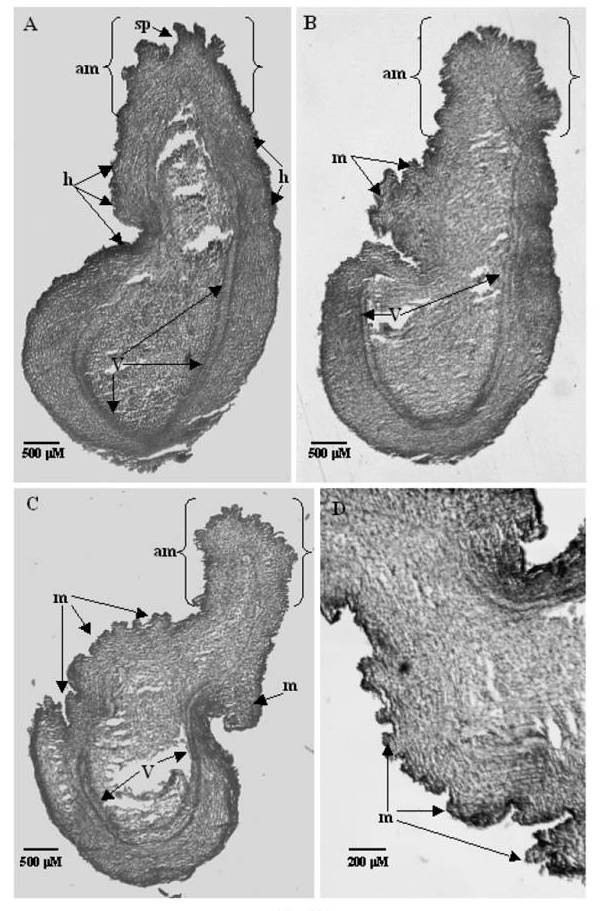
- Embryo axis:On microscopic examination of the longitudinal sections of the embryo axes cultured in 4.54 μM TDZ (Figure 2A) proliferation of morphogenic cells in the epidermal and subepidermal layers was noticed near the cotyledon attachment site. Vasculature of embryo axis was intact and differentiation of existing meristem was initiated at the plumular site as the lower concentration has not affected the differentiation of meristems. Longitudinal sections taken from the tissues preconditioned in 11.35μM TDZ (Figure 2B), showed more morphogenic activity in the cotyledon attachment site and in the epicotyl portion. Small meristematic protrusions all over the epicotyl and cotyledon attachment surface indicate high morphogenic activity. An absence of morphogenic activity in the hypocotyl and vasculature indicate that TDZ does not influence the cells of this part of the axis. In the tissues preconditioned to high TDZ concentration (13.12 μM), intense morphogenic activity and mass formation (Figure 2C & D) were noticed near the cotyledon attachment site and on the surface of the epicotyl.
5. Discussion
- TDZ, being a urea-based cytokinin, is non-degradable by cytokinin oxidase enzymes in plant tissues. Consequently, they are persistent in plant tissues, transforming them from cytokinin dependence to cytokinin autonomy. Makara et al. [14] reported that TDZ has a carryover effect that enabled shoots to continue proliferation on a hormone-free medium as the culture cycles increased. Hence plant species may need altered incubation periods for differentiation and morphogenesis. Therefore it was suggested by Guo et al. [9] that double stage culture procedure consisting of the culture of plant tissue on TDZ-medium for an appropriate period (usually 10 to 20 days) followed by a secondary TDZ free medium, is beneficial for shoot proliferation and/ or somatic embryogenesis. Hence, in Milletia, explants like Embryo axis, de-embryonated cotyledons were cultured in TDZ for 10 and 20 days and followed by a secondary TDZ free MS medium for 4-8 w.
5.1. De Novo Direct Response of Embryo Axis in TDZ Media
- In all the concentrations of TDZ tested, bud-like structures were more in 20 days TDZ preconditioned cultures, implying longer exposure in TDZ elicit a better de novo response. Adventitious bud like structures was more at the region of the axis where the cotyledon was attached (Figure 1B & 1C). It is interesting to note that TDZ induced caulogenic buds near to the wounding site of Embryo axis. Wounding induced meristematic activity had been very well documented in the species like Kandelia [22], black gram [1], tamarind [18] etc. The entire epicotyl portion of embryo axis became morphogenic (Figure 1D & 1E) with an increase in either concentration or exposure of TDZ. Hypocotyl portion of embryo axis showed degeneration of tissues. For differentiation of these bud-like structures from embryoaxis explants, various efforts were takenon the basis of literature. But all efforts taken remained unsuccessful.This irreversible suppression of differentiation in the TDZ preconditioned embryo axis cannot be explained with the present state of knowledge. A similar observation was reported in chestnut [25]. In chestnut, only a few embryo axes explants regenerated buds after culture on TDZ medium. The authors suggested that possibly the high cytokinin activity of TDZ causing disorganization of the meristematic zone.
5.2. Culturing of Proximal Segment of De-embryonated Cotyledon Explants for Histological Comparison
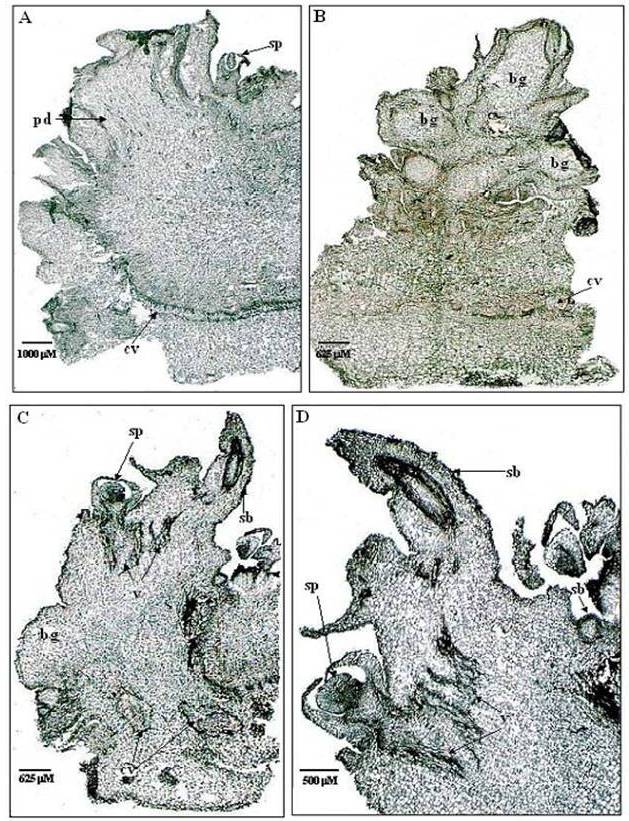
- Unlike embryo axis explants, the TDZ could induce and differentiate the caulogenic buds from the proximal segments of de-embryonated cotyledon into plantlets as reported earlier [29]. Histological examinations of these buds were carried out to confirm its de novo origin and about the extent of differentiation.
5.3. Histological Analysis
- The embryo axis explants pre conditioned in the lower concentration of TDZ (4.54 μM), showed differentiation of buds only after 4w of culture in TDZ free MS medium (Figure 2A). Shoot differentiation was evident in after 10 days of culture in TDZ free MS medium (Figure 3A). Possibly the carryover of TDZ might play a role in the delay of differentiation. At 11.35 μM of TDZ, the embryo axis explants showed meristematic masses triggered around the wounded site in the sub-epidermal layer and also the proliferation of apical meristem was also observed (Figure 2B). In cotyledon explants, differentiation of shoot primordia was quite evident from the section with the formation of new vasculature (Figure 3C). An overall proliferation of cells marked intense meristematic activity. At higher doses of TDZ (13.12 μM), the shape of the embryo axis got changed due to the proliferation of cells at epicotyl region. Hypocotyl region was not responsive to TDZ induction after 4w culture in TDZ free MS medium (Figure 2C). Unlike embryo axis, the entire cotyledonary segment responded to TDZ induction over time and resulted in a huge number of meristematic buldges after 10 days of culture in TDZ free media (Figure 3B). Shoot primordial differentiation might follow after 4w of culture in TDZ media as observed in Figure 3C.In Kandelia [22], it was reported that wound-induced meristem is responsible for shoot formation. These meristems are always in association with the vascular tissue, it is likely that material such as additional nutrients or a higher concentration of certain metabolites may be present in or near the vascular bundles which could induce shoot organogenesis from the wound-induced meristem cells. Hence, the bud primordia have endogenous in origin.In our study proliferation was observed in the subepidermal layers unlike deep into the vasculature. Mehta et al. [18] reported the development of abundant meristematic zones in the epidermal and subepidermal layers in tamarind. Similar observations were reported in peanut seedlings [31], Cercis Canadensis [7] and Phaseolus vulgaris [15], Pepper [19]. In embryo axis explants at higher doses TDZ (Figure 2C) meristematic activity proceeded toward teratological protuberances as in the case of Capsicum annuum [19], that lead to disorganized organogenesis with failed differentiation. TDZ is the most potent GR in elucidating in vitro response with all types of explants. Among the seedling explants, cotyledonary explants with intact embryo axis respond better in culture [21]. Similarly embryo axes with attached cotyledon explants respond better in culture [1]. De-embryonated cotyledon explants also respond to TDZ, undergoes adventitious regeneration pathway in Pongamia/ Milletia [29] and in many other plant species [21, 2], resulting in the formation of plantlets. Possibly, Cotyledonary tissues might have provided factors for initiation of normal meristematic zone upon TDZ induction. However, the embryo axis explants without cotyledon attachment upon TDZ induction showed a anomalous response. It undertakes direct adventitious organogenesis pathway upon TDZ induction, to produce caulogenic bud-like structures that fail to regenerate/differentiate into shoots.Histological studies made it clear that bud-like structures induced by TDZ in embryo axis explants are not truly caulogenic buds and so fail to differentiate even at lower concentrations TDZ. It is evident that TDZ could not induce normal meristematic activity in embryo axis explants but only pseudobuds. The unique response of TDZ with embryo axis explants need to be studied in detail. It is known TDZ promoted organogenesis comprises a metabolic cataract including primary signaling event, storage, a passage of endogenous signals and iron in a plant cell, a system of secondary messengers and a simultaneous stress response which may or may not be established as organogenesis [10]. In spite of its high potency as a phytohormone and more than thirty years of research, the factors that regulate TDZ action in tissues and its exact biological role is still a ambiguity.
6. Conclusions
- In Pongamia/ Milletia, we could demonstrate by histological examination that TDZ showed normal signaling pattern with the formation of adventitious caulogenic buds and following differentiation in to shoot, with de-embryonated cotyledonary explants. In embryo axis explants, TDZ failed to trigger normal adventitious signaling pattern, instead resulted in adventitious bud-like structures. This could be due to anomalous signaling that caused meristematic aberrations that proceeded toward teratological protuberances, resulting in lack of shoot primordial development. These changes could be identified by histological examination precisely when morphological observations provide no clue. It is well known in the literature that TDZ promotes bud induction but inhibit its differentiation due to its carryover effect [14] at higher concentrations. But we report in our study that even at lower concentrations TDZ, it causes abnormal meristematic protuberances resulting in aberrant caulogenic buds/pseudo buds, though looks morphologically normal but histologically demonstrated abnormal, thus an absence of differentiation into shoots. This could be due to altered TDZ signaling that resulted in defective meristematic activity in embryo axis explants without cotyledon attachment. A detailed study is needed to understand the unique response of TDZ with embryo axis explants Role of TDZ in promoting organogenesis comprises a metabolic cataract including primary signaling event, storage, passage of endogenous signals and iron in plant cell, a system of secondary messengers and a simultaneous stress response which may or may not be established as organogenesis [10]. In spite of extensive research, the biological role of TDZ is still a mystery and the factors that regulate TDZ activity in tissues especially embryo axis is yet to be studied.
ACKNOWLEDGEMENTS
- We acknowledge CSIR, India for the Research fellowship awarded to the author Sujatha Raman (K.Sujatha).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML