-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2018; 8(1): 1-7
doi:10.5923/j.ijmb.20180801.01

Laboratory Assessment of the Toxic Efficacy of Selected Seed Oils against the Emergence of Cowpea Weevil (Callosobruchus maculatus F.) on Stored Cowpea (Vigna unguiculata L. Walp)
1Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
2Department of Biology, Shehu Shagari College of Education, Sokoto, Nigeria
Correspondence to: M. M. Yahaya, Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Toxic effect of five oil types, manually extracted from kernels of neem, cotton, groundnut, castor and desert dates and pair wise mixtures in 1:1 ratio to obtain tem combinations, were tested against the emergence of Callosobruchus maculatus (F.) from treated cowpea seeds. Stored cowpea (Vigna unguiculata L. Walp) of Sokoto local and Kananado varieties were separately treated with varying dosages of straight and mixtures of the extracted oils (0.5, 1.0, 2.0, 3.0, and 4.0ml/50g seed) in Petri-dishes (9 x 9cm) before infested with 10 pairs of adults C. maculatus maintained alongside 50g untreated seeds in three replicates. Observation for insect emergence from the treated and untreated cowpea seeds was made 5 weeks post-infestation by recording the number of adults from each sex in each Petri-dish containing seeds. Generally, the emergence of the insects from the treated seeds was observed to be indirectly proportional to the varying dosages of the oils applied while straight and mixtures of neem oil proved to be more efficacious. Differences in emergence among the treatment means and untreated were significant (P<0.05) in most of the cases. Comparatively, less number of adults emerged out from both the treated and untreated Kananado seeds than from Sokoto local, which may imply that in addition to the effects of oils, the variety is resistant to the attack of bruchids.
Keywords: Callosobruchus maculatus, Cowpea, Emergence, Seed oil, Treatments
Cite this paper: M. M. Yahaya, B. Sulaiman, Laboratory Assessment of the Toxic Efficacy of Selected Seed Oils against the Emergence of Cowpea Weevil (Callosobruchus maculatus F.) on Stored Cowpea (Vigna unguiculata L. Walp), International Journal of Modern Botany, Vol. 8 No. 1, 2018, pp. 1-7. doi: 10.5923/j.ijmb.20180801.01.
Article Outline
1. Introduction
- Callosobruchus maculatus (F.) also known as bruchid or cowpea weevil, is the primary pest of stored cowpea (Vigna unguiculata (L.) walp). It is polymorphic and has been confused with C. analis, which has non serrate antennae, with C. chinensis which has a more angular outline and with C. subinaetatus which lacks the dark spots on the elytra and is larger [1]. Eggs are laid by the female bruchids on cowpea seeds or pods and the hatched larvae bore into the seed where the entire immature life is spent. The adults emerge out through circular holes they made leaving the seeds damaged. Under favorable conditions, up to 18 adult bruchids can emerge from a single seed [2] and each window left means that one-tenth (1/10) of a seed has been lost [3]. A global conservative estimate puts grain legume losses during storage at between 15-25 percent annually [4]. Damage of up to 40 percent of cowpea in storage has been recorded in several parts of Africa [5] but C. maculatus particularly accounts for over 90 percent of the damage [6]. There is no surprise to this impact of damage because amongst the insect species that attack the pods of cowpeas in the field, only the bruchids survive in the store and among the latter; only C. maculatus does well [7]. Infestation of postharvest cowpeas by bruchids also lead to loss in weight and quality of seeds, discoloration, changes of flavor, mould formation, reduced nutritional value due to lowered protein levels and poor germination of seeds due to embryo damage [8, 9]. Another aspect of concern aside from contamination of seeds by dead insects, pupae and larval cocoons is that, their integument has been found to contain various carcinogenic compounds such as ethyl, methyl and methoxy quinines which cannot be denatured by boiling or baking [10]. To control the menace of infestation of beans in storage, synthetic pesticides remain the most desired tool, but the steady increase in the cost, hazards from chemicals and development of resistance to the pesticides by pests, made it necessary to consider alternative measures of control that are effective, safer and relatively resistant-free. Resistance to pesticides was not serious before the early 1940's when raw plant materials were the principal agents of pest control [11]. Scientists estimate that pesticides are reducing crop yield by one-third through impaired nitrogen fixation, thus, opined that, pesticides and other contaminants should be substituted by yield enhancing benefit of well managed, organic farming system [12]. In many areas of the world locally available materials are widely used to protect stored produce against damage by insect infestation. Small-scale farmers throughout Nigeria are no exception in admixing wood ashes with cereal and pulse grains that they select and store for use as seed. Vegetable oils, leaves and twigs of certain plants and even dried sand and table salt are used for the same purpose. Over 2000 species of plants produce chemicals that have repellent or controlling properties [13]. Among other reports, seed oil of rubber plant, coconut, palm kernel and some other essential oils were applied on cowpeas in storage to control C. maculatus infestation [14-17].
2. Objectives
- The current study reported the efforts made to suppress emergence of C. maculatus from treated cowpea seeds with oils extracted from five locally available plants. It has also gone further from the traditional practice of straight oil application on cowpeas to combination of the oils in order to compare the effect against the targeted cowpea weevils.
3. Materials and Methods
3.1. Oil Extraction
- Five oil types used in the study are neem oil, cotton oil, groundnut oil, castor oil and desert dates oil respectively extracted from the kernels of Azadirachta indica (A. Juss), Cossypium hirsutum (L.), Arachis hypogea (L.), Ricinus communis (L) and Balanites aegyptiaca (L. Del.). In each case, the seeds were decorticated to separate the kernels which ware then pan grilled on fire and pound to form a paste. The paste was hand-pressed to give off the oil. Mixtures of the oil extracts were also prepared in 1:1 ratio to obtain ten separate combinations which were also screened. Manual extraction of oil was employed because low-income farmers use oil as such in protecting cowpea in storage.
3.2. Cowpea Varieties Used
- Two cowpea varieties susceptible to bruchid attack were selected; the Sokoto local that has small seeds with rough white seed coat and a brown eye, one hundred seeds weigh 13.9g and Kananado having large seeds with rough white seed coat, black eye and weigh 16.7g for hundred seeds. Adequate amount of seeds for each variety was sterilized in a deep freezer for two weeks to disinfest any prior infestation by cooling [13]. The seeds were then dried under ambient laboratory conditions for another two weeks and kept as stock.
3.3. Rearing of the Insects
- The adults of C. maculatus were obtained from reared cultures in the laboratory maintained on cowpea at 30±2°C and 80±5% RH. They were fed on sterilized cowpeas in four glass jars (each measuring 350ml) under similar conditions till a new generation of adults emerged. Total developmental period from eggs to adults under above conditions was between three to four weeks. The new generation adults were then used as mother stock for experimental purpose.
3.4. Experimental Protocol
- 150g of sterilized seeds of Sokoto local variety ware obtained from the stock and thoroughly mixed with 1.5ml neem oil. The seeds were then divided into 3 separate Petri dishes (9x9cm), each containing 50g seeds treated with 0.5ml oil. The fourth Petri dish with 50g untreated seeds was also obtained as control. Ten pairs of approximately 2-day-old adult bruchids were obtained from the mother stock using camel hair brush and released in each Petri dish containing seeds. All the Petri-dishes were covered with muslin cloth tied by rubber band to prevent insect escape. Female insects are recognized by the presence of four maculated elytral spots and males with plain, less distinctive spots [18]. All the Petri dishes containing seeds were kept under laboratory conditions while the insects introduced were allowed to lay eggs till they died (14 days later) and then were removed. Observation on emergence was made 5 weeks post infestation by recording the number of adult insects (sex wise) that emerged from oil treated and untreated seeds. Similar experiments were conducted by applying increased dosage (1.0, 2.0, 3.0 and 4.0ml) of the same and other extracted oils, as well as when Kananado variety was used. Likewise when applying the ten combinations of the oils for each 50g of seeds. The results obtained were statistically analysed using ANOVA test and comparison of treatment means was based on using Duncan’s Multiple Range Test.
4. Results and Discussion
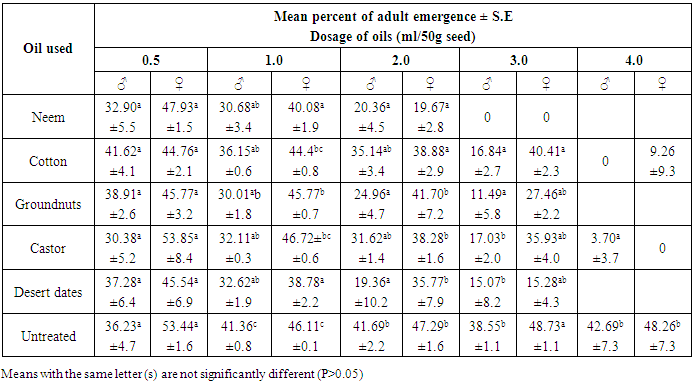
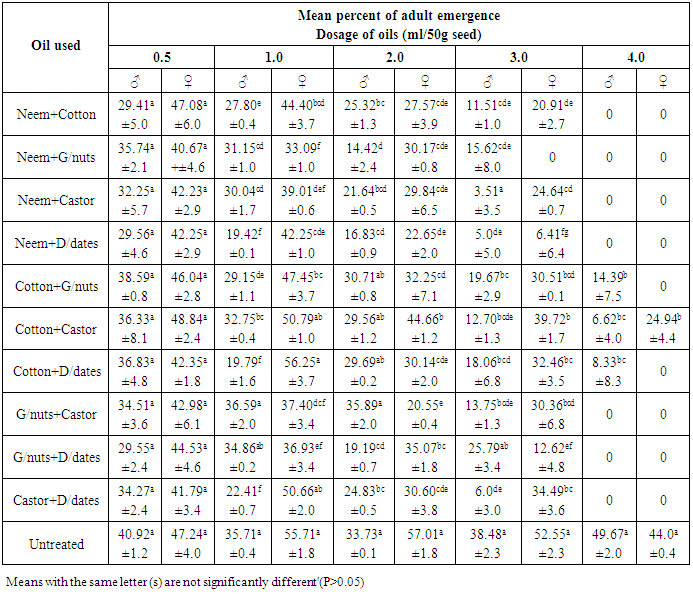
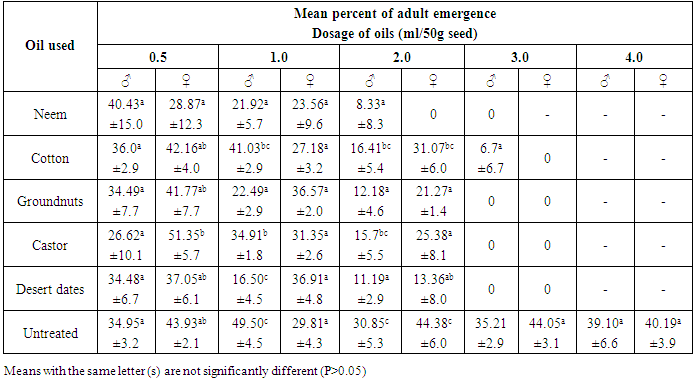
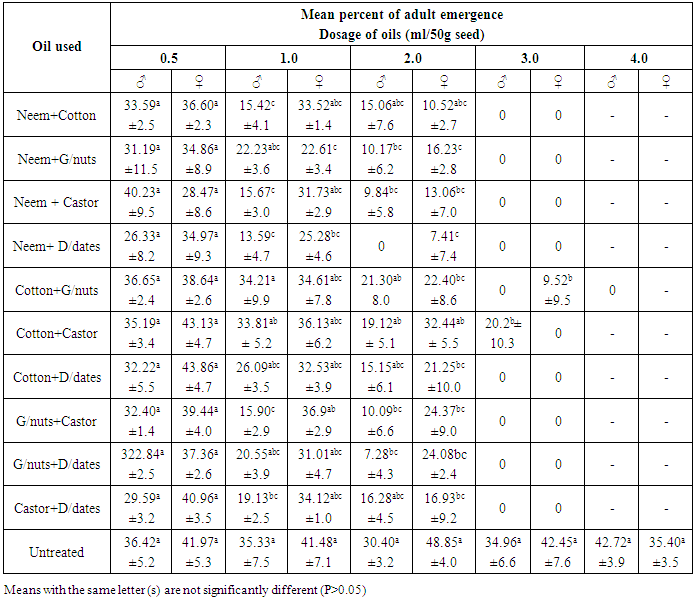
- The progeny of C. maculatus started to emerge from the treated and untreated cowpea, 31 days after introducing the parent bruchids and lasted for 5 days giving a range of 31-36 days as the development period. This range was at variance with 18-25 days reported [18] on the bruchids emergence from seeds treated with essential oils but similar to those reported at 21°C and 70% RH [19, 7].It was observed that, emergence of bruchids was not significantly (P>0.05) affected when lowest dosage (0.5ml/50g seed) of straight and mixtures of oils were used on cowpea seeds. However, neem, groundnut, castor and desert dates oils effectively reduced the emergence among male bruchids from the seeds of Sokoto local variety treated with 1.0ml/50g seed, while only neem and desert dates oils affected that of females under the same dosage (Table 1). The efficacy of neem oil in this regards, became more pronounced at the dose of 2.0 ml/50g seed while at 3.0 and 4.0ml/50g seed no emergence of bruchids occurred. But, all other oils remained insignificant for reducing the emergence of both male and female bruchids compared to control, except when the seeds were treated with 4.0ml/50g seed. Among the different combinations of oils, the mixtures of neem + cotton, neem + groundnut, neem + castor and neem + desert dates oils significantly (P<0.05) reduced the emergence of both males and females C. maculatus on treated seeds with 1.0, 2.0 and 3.0ml oil, but emergence was inhibited completely at 4.0ml/50g seed (Table 2). The rest of the oil mixtures were observed with varying effects on emergence among the sexes at 1.0 and 2.0ml/50g seed application. Apart from the mixture of groundnut + desert dates oils which was not significant in affecting the male emergence from seed treated with 3.0ml/50g seeds, all other mixed oils applied were very effective at 3.0 and 4.0ml/50g seed (Table 2). On Kananado variety of cowpeas, treatment with neem, groundnut, castor and desert date’s oils at 1.0ml/50g and above significantly (P< 0.05) reduced the emergence among male bruchids and at 2.0ml/50g seed among females (Table 3). Of these oils only that of castor and cotton didn't affected the emergence among males at 2.0ml/50g seeds but both affected the emergence significantly at 3.0 and 4.0ml oil compared to emergence from untreated seeds. Treatments of seeds with the mixed oils showed that, only neem + desert dates oils applied at and above 1.0ml/50g significantly reduced the emergence of adults of both sexes, but mixtures of neem + groundnut, neem + castor, groundnut + castor and groundnut + desert dates oils also were significantly effective at and above 2.0ml/50g seed. The remaining mixed oils were effective when applied on seeds at 3.0 and 4.0ml/50g seed (Table 4). These varying effects on adult C. maculatus emergence as observed in the present study could probably be due to some seeds that might not have been coated with oil uniformly. It was [20] who demonstrated the importance of a uniform coating of oils on bean seed. However, all the oils used were effective, and it was possible that, the oils formed physical barrier and thus prevented the entrance of some larvae in the seeds thereby reduced the subsequent emergence of adults from the treated seeds. Scholars [21] have shown that eggs laid on cowpea had an increase in oxygen uptake between the first to seventh days after oviposition and if oils reduced the oxygen uptake, eggs will starve. Cowpeas contain 1.18g of lipids, 26.53g of proteins and 54.99g of carbohydrates per 100g of dry matter [22], the admixture of cowpea with seed oils would raise the level of lipids and whose over consumption by the larvae would alter the balance between essential and non-essential fatty acids, resulting in an increase requirements for the essential acids for those larvae that succeeded in penetrating the seeds, and this would affect the larvae and then adults that would emerge out. It was also likely that, the oils used could have acted as antifeedants, which means that, the larvae dies through starvation before they reached maturity or adult stage. It was [23] who observed the similar reduced emergence of C. maculatus on cowpea treated with 8ml neem oil/kg seed. But [20] also reported similar significant reduction in the emergence of Zabrotes subfasciatus on beans treated with cotton seed and palm oils. Similarly, [24] also reported reduced emergence of C. maculatus on artificial black eye peas treated with peel oils of lemon, grape fruit, limes and tangerine. Though these authors attributed the action of vegetable oils to the chemical properties of the oils, they were not specific about the chemicals involved. However, neem oil possesses extraordinary gastotory repellent properties against the desert and migratory locust [25] while azadirachtin and salanin compounds isolated from neem oil possessed activity as feeding deterrent against striped cucumber beetle Diabrotica undecimpunctata [26, 27]. There was evidence of growth disruption, feeding inhibition, deterrence and outright mortality associated with neem based products on certain insect species [28]. Oil from cotton seed contains aromatic sesquiterpens dimmer and gossypol compounds [29, 30]. Gossypol compounds are believed to cause male sterility among many species of insects including bruchids [31]. Toxicity of these compounds against tobacco budworm (Heliothis virescence), pink bollworm (Pectinophora gossypiella) and boll weevil (Anthonomus grandis) has been established [32]. Thus, the compounds might have played roles in affecting the emergence of C. maculatus in this study. Groundnut seed’s glycerides contain certain allelochemicals comprising phytohaemagglutinins, protease inhibitors, and saponins [33, 34] all of which were reported toxic to C. chinensis [35] and C. maculatus [22]. It could be possible that the compounds might be present in the groundnut oil used in the present experiment and had an effect on reducing the bruchids emergence. The foliage of castor plant possesses insecticidal properties and the meals from its beans proved toxic to livestock [36]. Presumably, the oil extracted from the beans of castor might as well be toxic and that C. maculatus became vulnerable to it thereby caused reduced emergence. Different extracts of desert dates (including the oil) contain saponins [37] while the bruchids are unable to hydrolyzed saponins [38]. Moreover, the ovicidal and molluscicidal activities of desert dates oil have been reported [39]. The effect of seed oils on the emergence of C. maculatus was apparently greater on kananado variety of cowpeas than on Sokoto local variety. Present observation revealed that, the testae of the seeds of Kananado are tough and thick, suggesting an additional advantage when coupled with the action of the seed oils for affecting the insect’s emergence from treated seeds with oil. A report [28] has shown that, the efficacy of vegetable oils is quite variable, but they should work well in combination with resistant cultivar.
|
|
|
|
5. Conclusions
- All the seed oils tested in this study did not significantly reduced the bruchids emergence when the lowest dosage was applied (0.5ml/50g seed). Application of higher dosages (2.0ml/50g seed and above) of each oil reduced emergence of both sexes of the bruchids. Neem oil was however, more efficacious in this respect when applied either as straight or in combination with other oils used. Adult emergence was observed to be indirectly proportional to the dosage of the oils applied. Lesser emergence of bruchids occurred on kananado seeds which might indicate resistant of the variety to the bruchid attack. The activity of seed oils against bruchids emergence can be well-utilized, while planning for more alternative control measures against the bruchid.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML