-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2016; 6(1): 10-17
doi:10.5923/j.ijmb.20160601.03

Evaluation of Secondary Metabolites in Callus and Tissues of Physalis peruviana
Islam I. Lashin , Mohammed H. Elhaw
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
Correspondence to: Islam I. Lashin , Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present paper deals with In-vitro callus induction and shoot regeneration in Physalis peruviana. Physalis peruviana belong to the family Solanaceae. The mature seeds cultured on Murashige and Skoog (MS) medium supplemented with different concentrations of 6-benzylaminopurin (BAP) singly or in combination with Naphthalene acetic acid (NAA). The best result for callus induction were obtained from seed with MS medium supplemented with 1.5 mg/L BAP + 0.5 mg/L NAA. These calli were subcultured on MS medium supplemented with various concentrations of BAP alone for regeneration.MS medium supplemented with 1 mg/l BA gave the highest number of shootlets regeneration with seed calli. In this study we use different tissue to determine the quantity and qualitative of alkaloids, glycosides, cardiac glycosides, saponins, phenol, sterol, tannins, flavonoids, diterpene and volatile oil.
Keywords: Physalis peruviana, Callus induction, Shoot regeneration and secondary metabolites
Cite this paper: Islam I. Lashin , Mohammed H. Elhaw , Evaluation of Secondary Metabolites in Callus and Tissues of Physalis peruviana, International Journal of Modern Botany, Vol. 6 No. 1, 2016, pp. 10-17. doi: 10.5923/j.ijmb.20160601.03.
Article Outline
1. Introduction
- The genus Physalis L. of the Solanaceae family, has approximately 120 species distributed throughout the planet’s temperate zones, from southern North America to South America, with its taxonomical diversity focus in Mexico, United States and Central America [1]. Physalis peruviana L. is the species most commonly found in Brazil. It is commonly used as folk medicine for treating cancer, leukemia, hepatitis, rheumatism, and other diseases [2, 3]. Major bioactive compounds of Physalis spp. such as physalins (B, D and F) [4, 5] and glycosides (such as myricetin-3-O-neohesperidoside) [6]. The fruit has been used as a good source of provitamin A, minerals, vitamin C and vitamin B-complex. The fruit contain 15% soluble solids (mainly sugars) and its high level of fructose makes it valuable for diabetics. Several compounds from P. angulata have been isolated and chemically characterized. A group of steroids, known as physalins, are found in P. angulata stems and leaves [7].For this above medicinal purposes, this plant is highly focused in many countries and pharmaceutical industries. Tissue culture plays an important key role for medicinal plants in rapid propagation and enhanced the production of secondary metabolites. Plant tissue culture technology offers excellent opportunity for conservation of economically important plant species. The capability to regenerate and propagate plants from cultured cells and tissues is one of the most exciting and useful aspects of in vitro cell and tissue culture [8].Callus obtained from these explants was greenish yellowish and very soft in nature [9]. Shoot induction, proliferation and maintenance for longer period of time is governed by different PGRs and culture conditions [10]. The highest regeneration of P. minima was obtained in the MS medium fortified with 2 mg/l BAP and 0.25 mg/l IAA [11]. Work done by [12] used node, internode and leaf explants Physalis peruviana L with various concentrations and combination of BAP and 2, 4-D for culture initiation and multiplication of shoots. In India, to evaluate different extraction processes on the basis of preliminary screening of the bioactive phytochemicals in the extracts, as well as two processed products (fresh fruit juice and squash) of cape gooseberry [13].Aim of this study to estimation the some secondary metabolites in different tissue, regeneration plant, callus from seed & leaf and fruits from mother plant of Physalis peruviana L.
2. Materials and Methods
2.1. Plant Materials
- Surfaces of Physalis peruviana L seeds were sterilized with 10% (v/v) sodium hypochlorite for 15 min. After rinsing with sterile distilled water, seeds were germinated on medium containing 1% (w/v) agar and MS medium [14] supplemented with 3% (w/v) sucrose and different concentration of growth regulator at 25 ± 2°C under 16 h photoperiod with fluorescent light.
2.2. Callus Induction
- Callus cultures were established from seed, leaf and stem explants from three week- old seedlings germinated in vitro. Callus culture were grown at 25±2°C with a photoperiod of 16 h fluorescent light in MS basal medium supplemented with different concentration of (BAP) and (NAA).
2.3. Shoot Regeneration
- After two weeks from callus formation were transferred to MS medium containing different concentration of BAP alone. The cultivars were incubated at 25°C in the 16/8 h (light/dark) photoperiod.
2.4. Preliminary Phytochemical Screening.
- v Preparation of the Alcoholic Extract for Further ScreeningAbout 100g of air-dried plants from regeneration plant, callus from seed & leaf and fruits from mother plant of Physalis peruviana were used for preparation of alcoholic extract. Powder of each part were refluxed with 2.5 L of 70% methyl alcohol for 6 hours, and then filtered. The residue powder was then washed several times with hot alcohol. The combined filtrates were concentrated under reduced pressure at 50ºC, and then used for the following tests: Tests for alkaloids, glycosides, cardiac glycosides, saponins, phenols, sterols, tannins, flavonids, amino acids, and volatile oil.
2.4.1. Test for Alkaloids [15]
- The alcoholic extracts for callus, regeneration plants and fruits of the plant were concentrated under vacuum till dryness. The dried extracts was dissolved in 2N-hydrochloric acid PH= 1.5 on a water bath, shaken and filtered, the obtained filtrates was shacked with chloroform to remove undesirable matters.The acidic aqueous layer was adjusted to alkaline PH= 9 with ammonia and the liberated alkaloid bases were extracted by chloroform till exhausted and then tested by Mayer’s and Dragendorrf’s reagents. The presence of precipitation or micro-crystal on a microscope slide indicated the presence of alkaloids.Reagents for Alkaloids [16]a) Wagner’s reagent (Potassium tri-iodide)
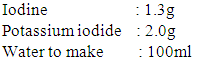 b) Dragendorrf’s reagent (Potassium bismuth iodide)* Solution (A): 1.7g of bismuth subnitrate and 20g tartaric acid were dissolved in 80ml water.* Solution (B): 16g potassium iodide were dissolved in 40ml water * Stock solution: 1:1 (v/v) mixture of (A) and (B) was freshly prepared for spraying.Spray reagent: 5ml of stock solution were added to a solution of 10g tartaric acid in 50ml water.
b) Dragendorrf’s reagent (Potassium bismuth iodide)* Solution (A): 1.7g of bismuth subnitrate and 20g tartaric acid were dissolved in 80ml water.* Solution (B): 16g potassium iodide were dissolved in 40ml water * Stock solution: 1:1 (v/v) mixture of (A) and (B) was freshly prepared for spraying.Spray reagent: 5ml of stock solution were added to a solution of 10g tartaric acid in 50ml water.2.4.2. Test for Glycosides
- a) Glycosides Test [17]To small amount of extract, add 1 ml water and shake well. Then aqueous solution of NaOH was added. Yellow colour appeared that indicates the presence of glycosides.b) Modified Borntrager’s Test: [17]Extracts were treated with Ferric Chloride solution and immersed in boiling water for about 5 minutes. The mixture was cooled and extracted with equal volumes of benzene. The benzene layer was separated and treated with ammonia solution. Formation of rose-pink colour in the ammonical layer indicates the presence of anthranol glycosides.
2.4.3. Test for Cardiac Glycosides
- a) Legal’s Test [17]Extracts were treated with sodium nitropruside in pyridine and sodium hydroxide. Formation of pink to blood red colour indicates the presence of cardiac glycosides.
2.4.4. Test for Saponins
- a) Foam Test: [18]The extract was diluted with 20 ml of distilled water and it was shaken in a graduated cylinder for 15 minutes. A 1 cm. layer of foam indicated the presence of saponins.b) Haemolysis Tests: [19]Add extract to one drop of blood placed on glass slide. Hemolytic zone appears.
2.4.5. Test for Phenols
- a) Ferric Chloride Test: [20] Extracts were treated with 3-4 drops of ferric chloride solution. Formation of bluish black colour indicates the presence of phenols.
2.4.6. Test for Phytosterols
- A few mls of the alcoholic extracts were evaporated till dryness. The residue was dissolved in 2ml chloroform and filtered, where the filtrate was subjected to:a) Salkwski Reaction’s [21]To one ml chloroform extract, conc. sulphuric acid was added slowly down the side of the test tube, where positive reaction was indicated by the formation of yellow colored ring changing to bloody red.b) Libermann-Burchard’s test [22] by adding to one ml chloroform extract, one ml anhydrous acetic acid followed by few ml of conc. sulphuric acid, poured carefully down the side of test tube, where blue, green, red, or orange colors that change with time will indicate a positive reaction.
2.4.7. Test for Tannins: [17]
- a) Lead Acetate Test To 5 ml of extract, add few drops of 10% lead acetate solution were added. Formation of yellow or red precipitate indicated the presence of tannins.b) Gelatin Test:To the extract, gelatin (gelatin dissolves in warm water immediately) solution was added. Formation of white precipitate indicated the presence of tannins.
2.4.8. Test for Flavonoids
- a) Schinoda’s Test: [23]Half ml of hydrochloric acid was added to an aliquot of aqueous extracts followed by few mg of magnesium turnings. A pink color indicated the presence of flavonoids.b) NaOH Test: [24]To 2-3 ml of extract, few drops of sodium hydroxide solution were added in a test tube. Formation of intense yellow colour that became colorless on addition of few drops of dilute HCl indicated the presence of flavonoids.
2.4.9. Detection of Proteins and Amino Acids
- Xanthoproteic Test: The extracts were treated with few drops of conc. Nitric acid. Formation of yellow color indicates the presence of proteins.
2.4.10. Steam Distillation of Volatile Oils: [16]
- Fifty grams of fresh plant were subjected to steam distillation to extract volatile oils.
2.5. Investigation of Total Active Materials
2.5.1. Estimation of Total Phenolic Content (TPC)
- The amount of total phenolic in extract was determined with the Folin Ciocalteu reagent. Gallic acid was used as a standard and the total phenolic was expressed as μg/mg gallic acid equivalent to (GAE). Concentrations of 10, 20, 30, 40 and 50 μg/ml of gallic acid were prepared in methanol. Concentration of 1mg/ml of plant extract was also prepared in methanol and 0.5 ml of each sample were introduced into test and mixed with 2.5ml of a 10 fold dilute Folin Ciocalteu reagent and 2ml 0f 7.5% sodium carbonate. The tubes were covered with parafilm and allowed to stand for 30 minutes at room temperature before the absorbance was read at 760 nm spectrophotometrically. All determination was performed in triplicate. The Folin Ciocalteu reagent is sensitive to reducing compounds including polyphenols. They produce a blue color upon reaction. This blue color was measured spectrophotmetrically [25, 26]. Line of regression from Gallic acid was used for estimation of unknown phenol content. From Standard curve of Gallic acid line of Regression was found to bey = 0.0013x + 0.056R2 = 0.9872(y) was the absorbance and (x) was the μg GAE/mg of the extractThus the goodness of fit was found to be good for selected standard curve. By putting the absorbance of test sample (y = absorbance) in line of regression of above mentioned GA.)
2.5.2. Estimation of Total Flavonoid Content (TFC)
- The amount of total flavonoid content in extract was determined by aluminum chloride assay through Colorimetric method. A 0.5ml aliquot of appropriately diluted sample solution was mixed with 2 ml of distilled water and subsequently with 0.15ml of a 5% NaNO2 solution. After 6 minutes, 0.15 ml of a 10% AlCl3 solution was added and allowed to stand for 6 minutes, then 2 ml of 4% NaOH solution was added to the mixture. Immediately, water was added to bring the final volume to 5ml, then the mixture was thoroughly mixed and allowed to stand for another 15 minutes. Absorbance of the mixture was determined at 510 nm versus prepared water blank. Rutin was used as standard compound for the quantification of total Flavonoid. Total flavonoid content was expressed as mg rutin/g dry weight (mg rutin/g DW), through the calibration curve of Rutin. All samples were analyzed in three replications [27, 28].y = 0.0012x + 0.0011R2 = 0.9986(y) was the absorbance and (x) was the μg rutin/mg of the extract thus the goodness of fit was found to be good for selected standard curve. By putting the absorbance of test sample (y = absorbance) in line of regression of above mentioned rutin).
2.5.3. Estimation of Total Tannins
- Gravimetric Method (Copper Acetate Method):This method depends on quantitative precipitation of tannin with copper acetate solution, igniting the copper tannate to copper oxide and weighing the residual copper oxide [29].Two grams of parts were separately extracted for about one hour with two successive quantities, each of 100 ml of acetone-water (1:1) and then filtered. The combined extract, in each case, was separately transferred into a 250 ml volumetric flask and adjusted for volume with distilled water. Each extract was quantitatively transferred to a 500ml beaker and heated till boiling, then 30ml of 15% aqueous solution of copper acetate was added with stirring. The precipitate of copper tannate was collected on ashless filter paper and the precipitate was ignited in a porcelain crucible (the crucibles were previously ignited to a constant weight at the same temperature). Few drops of nitric acid were added to the residue and reignited to constant weight. The weight of copper oxide was determined and the percentage of tannin was calculated according to the following correlation: Each 1g of Cuo = 1.305g tannins.
2.5.4. Estimation of Total Saponins
- 2g from regeneration plant, callus from seed & leaf and fruits of Physalis peruviana were dispersed in 20 ml of 20% ethanol. The suspension was heated over a hot water bath for 4 hours with continuous stirring at about 55°C. The mixture was filtered and the residue was re-extracted with another 200 ml of 20% ethanol. The combined extracts were reduced to 40 ml over water bath at about 90°C. The concentrate was transferred into a 250 ml separating funnel and 20 ml of diethyl ether was added and shaken vigorous. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated and 60 ml of n-butanol was added. The combined n-butanol extract were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation, the samples were dried in the oven to a constant weight. The saponins content was calculated in percentage according to [30, 31].
2.5.5. Estimation of Total Alkaloids (Gravimetric Method)
- About (2g) of regeneration plant, callus from seed & leaf and fruits of Physalis peruviana were extracted with 90% ethanol till exhaustion (tested with Mayer's reagent). The alcoholic extract of the plant was concentrated under reduced pressure until dryness at a temperature not exceeding 40°C, acidified with HCl (3%), and filtered; the filtrate obtained was extracted with chloroform to remove acid alkaloid portion. The acidic aqueous layer was adjusted to alkaline media with ammonia and the liberated alkaloid base portion was extracted with chloroform till exhaustion (tested by Mayer and Dragendorrf’s reagents). The chloroform extract was filtered over anhydrous sodium sulfate and evaporated under reduced pressure till dryness, then weighed it to calculate the percent w/w. [15]
3. Results and Discussion
3.1. Callus Induction
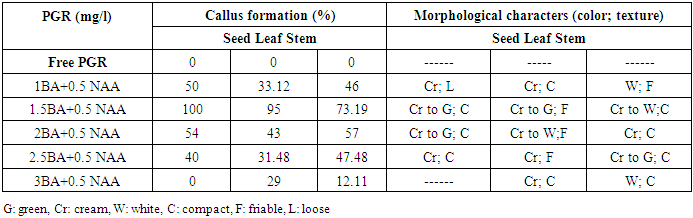
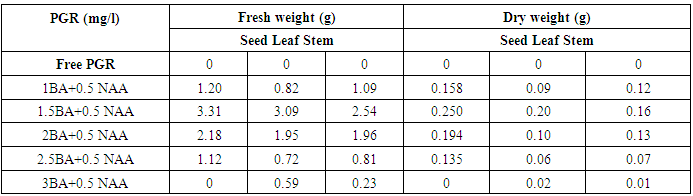
- This part of study aimed to record the various responses for callus formation using three explants (seed, leaf and stem) of Physalis peruviana L cultured five weeks on MS medium supplemented with different combinations of (BAP) and (NAA).The obtained results in table (1) and figure (1) revealed that the callus formation was varied depending on the type of explants and concentration of growth regulators used. The obtained data show that no callus was observed with any explants cultured on MS medium free of growth regulators.
|
|
3.2. Shootlets Regeneration
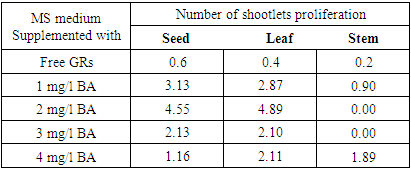
- In vitro shoot induction from callus of Physalis peruviana L on MS medium supplemented with various concentrations of BA. The first shoot induction was observed after 2 weeks in MS media supplemented with 2mg/l BA. The obtained results in table (3) and figure (1) revealed that leaflet calli cultivated on MS medium supplemented with 2 mg/l BA gave the maximum number of indirect shootlets regeneration (4.89). In addition, MS medium supplemented with 1 mg/l BA gave the highest number of shootlets regeneration with seed calli (3.13). Similar to our results, [35] observed that number of shoot per explants in potato increase with cytokinine concentration up to 5 units and then decreased.
|
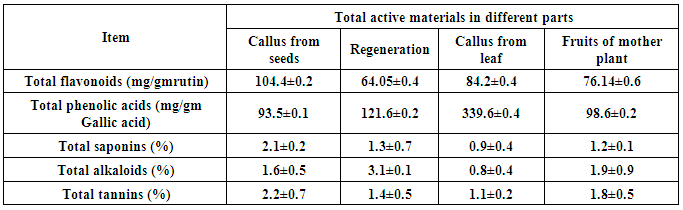
3.3. Determination of Total Phenols, Flavonoids, Alkaloids, Saponins and Tannins
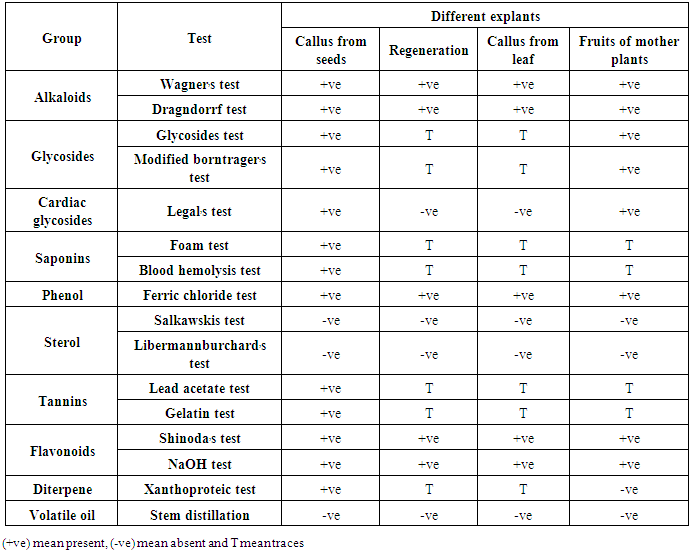
- Tissue culture has been suggested as a feasible technology for the production of many plant secondary metabolites. The presented data (Table 4) revered to the phytochemical screening in regeneration plant, callus from seed & leaf and fruits from mother plants of Physalis peruviana. In this study we use different tissue to determine present or absent of alkaloids, glycosides, cardiac glycosides, saponins, phenol, sterol, tannins, flavonoids, diterpene and volatile oil. The callus from seed showed better result for preliminary phytochemical screening than the regeneration plant, callus from leaf and fruits. Preliminary phytochemical screening of plant extract has been reported in several medicinal plants [36]. All the phytochemical components detected were known to support bioactive activities in medicinal plants [37].
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML