-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2015; 5(2): 38-46
doi:10.5923/j.ijmb.20150502.03

Crop Protection against Root Rot Fungi by Combined Effect of Homeopathic Drugs and Microbial Antagonists
Asma Hanif, Shahnaz Dawar
Department of Botany, University of Karachi, Karachi, Pakistan
Correspondence to: Asma Hanif, Department of Botany, University of Karachi, Karachi, Pakistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The objective of this research was to reduce economic losses result from root rot fungi by improving plant growth and control the plant pathogenic fungi to a low and safe level. Seeds of okra, sunflower, mung bean and mash bean treated with homeopathic drugs such as Arnica montana and Thuja occidentalis @ 50 and 75% v/v concentrations (prepared from 30C) along with microbial antagonists namely, Trichoderma harzianum, Paecilomyces variotii, Bacillus subtilus and Rhizobium meliloti drenched in soil which enhanced the plant weight and height but also reduced the Fusarium spp, Rhizoctonia solani and Macrophomina phaseolina colonization as compared to alone treatments. Highest number of nodules was observed on mash bean and mung bean seeds when treated with 75% v/v concentrations by both homeopathic drugs and soil were drenched with R. meliloti. Off the microbial antagonists used, T. harzianum together with treated seeds @ 75% v/v concentrations by both drugs showed better plant productivity and completely suppressed root rot fungi of test plants.
Keywords: Homeopathic drugs, Antagonistic fungi and bacteria, Root rot fungi and crop plants
Cite this paper: Asma Hanif, Shahnaz Dawar, Crop Protection against Root Rot Fungi by Combined Effect of Homeopathic Drugs and Microbial Antagonists, International Journal of Modern Botany, Vol. 5 No. 2, 2015, pp. 38-46. doi: 10.5923/j.ijmb.20150502.03.
1. Introduction
- Root rot diseases caused by soil borne pathogens considered as the most important diseases of many crops. Root rot pathogens including Fusarium spp., (produces wilt, root and stem rot diseases on a broad range of plants), Rhizoctonia solani Kühn (produces damping off of seedling, wilt, root and seed rot on over 2000 spp of plants), Macrophomina phaseolina (Tassi) Goid (produces charcoal rot, root and stem rot, seedling blight on 500 plant species) which decline yield of crop and reduced market values in many worldwide countries (Ghaffar, 1992; Benhamou et al., 1994; El-Mougy, 1995; Wheeler & Rush, 2001; Tanina et al., 2004). Extensively used of pesticides and other chemicals developed concerned of environment and health in finding the substitute technique for disease management of crop plants (Papavizas & Lumsden, 1980). Homeopathic drugs involves in biological processes of plants due to the secondary metabolites production which act as an environmental friendly without producing toxicity and leaving no residue (Bonato & Silva, 2003). Different formulations have been used to control soil borne pathogens like suspensions of bacteria (Paulitz et al., 1992), fungal spores (Harman et al., 1980), and powdery preparations of fungal mycelium (Latunde-Dada, 1993). Several studies have been conducted by additions of microbial antagonist in the soil it suppressed the population of soil borne plant pathogens and plant parasitic nematodes (Park, 1989; Saleem et al., 2000). Microbial antagonist such as Trichoderma spp., Paecilomyces lilacinus, Verticillium chlamydosporium, Bacillus spp., Stachybotrys atra, Pseudomonas aeruginosa, Gliocladium virens, have potential to decreased the colonization of root rot pathogens and also improved the plant growth (Rodriguez- Kabana et al., 1984; Lumsden & Locke, 1989; Izhar et al., 1995; Lewis et al., 1996; Bajwa et al., 2003; Abdel-Monaim, 2014). Antagonistic microbes have an ability to control soil borne pathogens by secretion of chitinolytic enzymes and production of mycoparasitism inhibitory compounds (Haram et al., 1996). These mycoparasitism inhibitory compounds might be the reason for the controlling of soil borne pathogens (Bari, 2001). Howell (2003) reported that due to chitinase enzyme it caused breaking of β-glucan, chitin and polysaccharides of fungal cells destroying pathogen. Such microbial antagonists build a protective layer around the roots of plants which prevent the entering of soil borne pathogen (Weller, 1988). Present research was conducted to explore the mutual relationship of homeopathic drugs along with microbial antagonist in the inhibition of root rot fungi.
2. Material and Methods
- Collection and population of antagonists: Cultures of Paecilomyces variotii (Pv-14), Trichoderma harzianum (Th-60), Bacillus subtilus (Bs-13) and Rhizobium melioti (Rm-19) were obtained from the Karachi University Culture Collection (KUCC). Antagonistic fungi (P. variotii and T. harzianum) were grown on PDA medium whereas antagonistic bacteria (B. subtilis and R. meliloti) were grown on nutrient broth medium and yeast extract mannitol agar (YEMA) respectively, incubated at 28-30°C for 24-96 hours depending upon population growth. Bacterial colonies (B. subtilis and R. meliloti) growing on Petri plates were counted and multiplied by the dilution factor which gave CFU/ml of bacteria (Yadav et al., 2010). Fungal population (P. variotii and T. harzianum) was determined by using serial dilution technique given by Aneja (2001).Seed treatment: Seeds of mung bean (Vigna radiata (L.) R. Wilczek. cv. NM-2006), okra (Abelmoschus esculentus (L.) Moench cv. Arka anamika), sunflower (Helianthus annuus L. cv. Hysun-38) and mash bean (Vigna mungo (L.) Hepper cv. NM-97) were treated with Dr. Willmar Schwabe homeopathic drugs like Arnica montana and Thuja occidentalis with the concentrations of 75 and 50% v/v (prepared from 30C) respectively whereas, seeds treated with sterilized water served as control for about 10-15 mins. and dried aseptically. In vitro: Pots experiment was conducted at the screen house bench of Botany department (Karachi University) in natural sunlight in a randomized design. Soil used for experiment was sandy loam containing; 74% sand, 9% clay and 17% silt (Gee & Bauder, 1986) of pH 7.6 (Brady, 1990), organic carbon 4.10% (Sparks, 1996), water holding capacity 38% (Keen & Rakzowski, 1922) and total nitrogen 0.83% (Mackenzie & Wallace, 1954) were placed in plastic pots containing 300g (8 cm diameter). Soil consist of natural infestation having R. solani 27% (Wilhelm, 1955), 8-11 sclerotia g-1 of M. phaseolina (Sheikh & Ghaffar, 1975), Fusarium spp 3700 cfu g-1 (Nash & Synder, 1962). Five treated seeds of mung bean, mash bean, sunflower and okra with different concentrations of homeopathic drugs were sown in pots respectively. Soil was drenched with 5 ml suspension of five day old cultures of P. variotii (24 ×10-6 conidia/ml), T. harzianum (72 × 10-7 conidia/ml) and 48h-old cultures of B. subtilus (14 × 10-8 cells/ml), R. meliloti (22×10-8 cells/ml). Treatments were replicated thrice and soil without antagonist suspension and untreated seeds served as control. Regularly water was given till the plants fully grown and then uproots it after one month.Isolation of root rot fungi: After careful uprooting, growth parameters like plant height, weight and number of nodules were recorded. Roots were cautiously washed in running tap water to remove soil particles and each root was cut into five pieces. These root pieces after surface sterilization with 1% Ca(OCl)2 for 5 mins., dried in blotter paper under laminar hood and transferred on poured potato dextrose agar (PDA) medium supplemented with antibiotics (penicillin @ 100,000 unit/L and streptomycin @ 200 mg/L) to inhibit the growth of bacteria. Incubate the Petri plates for one week at room temperature (27-33°C) and colonization of root rot fungi was recorded from each root segment.Statistical analysis: Data were analyzed by one way analysis (ANOVA) as per experimental design separately followed by the least significant difference (LSD) test at P = 0.05 according to Sokal & Rohlf (1995).
3. Results
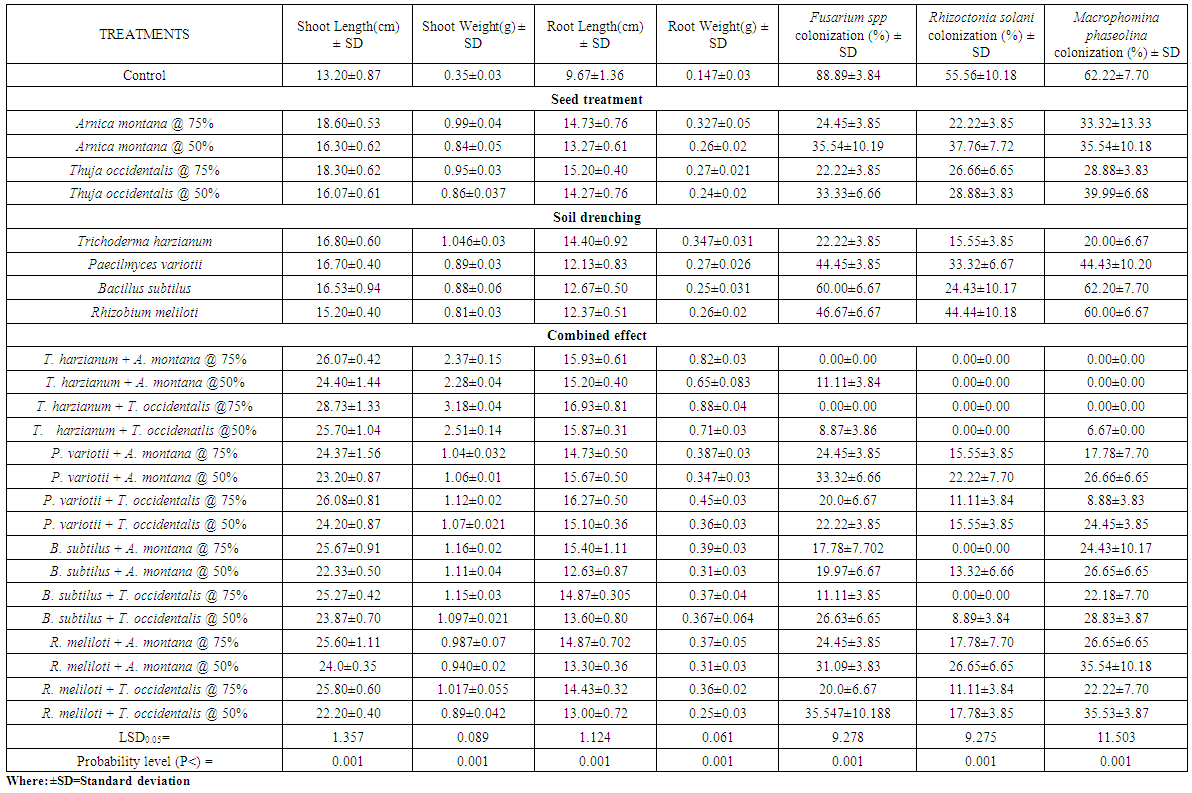
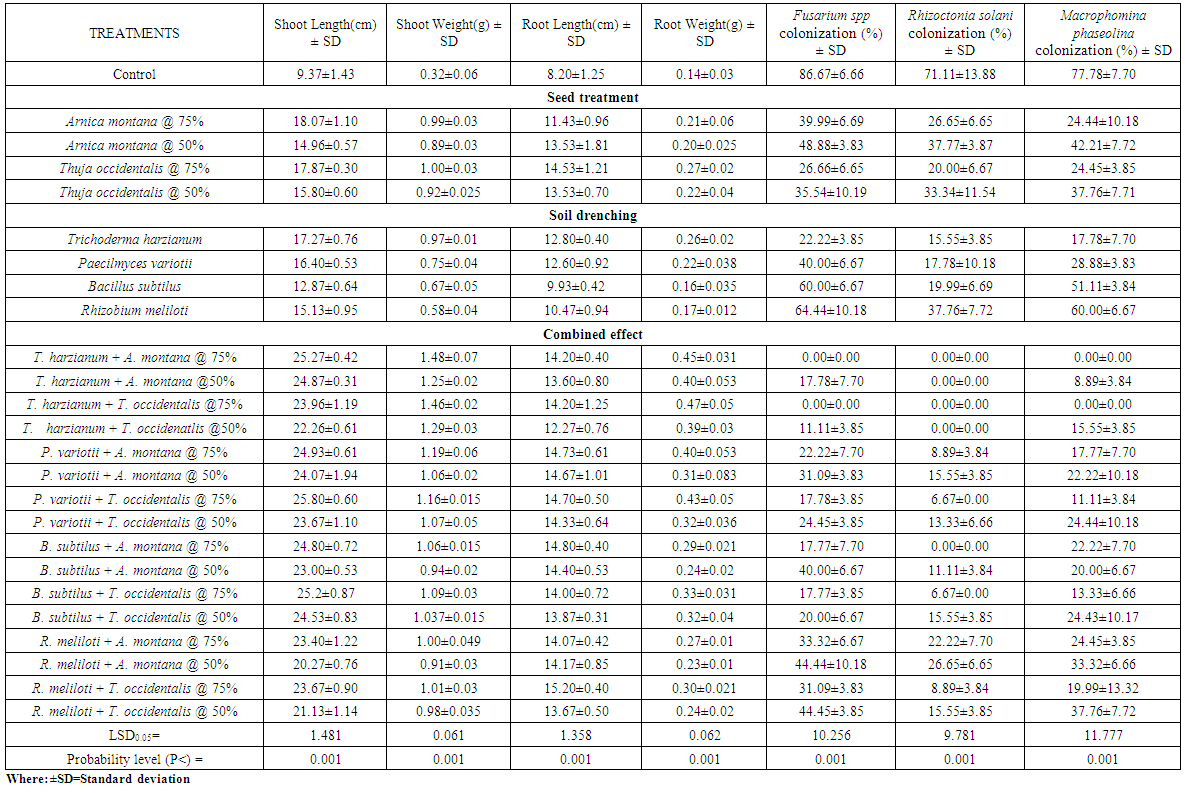
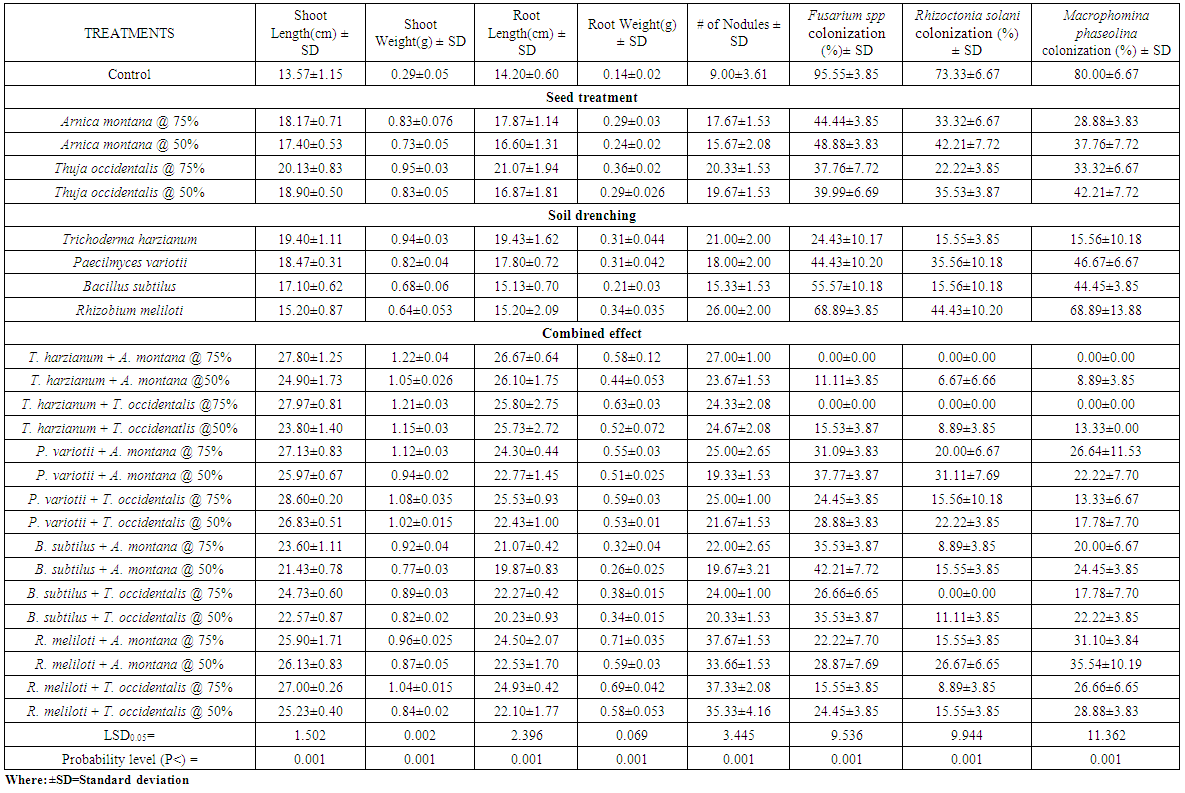
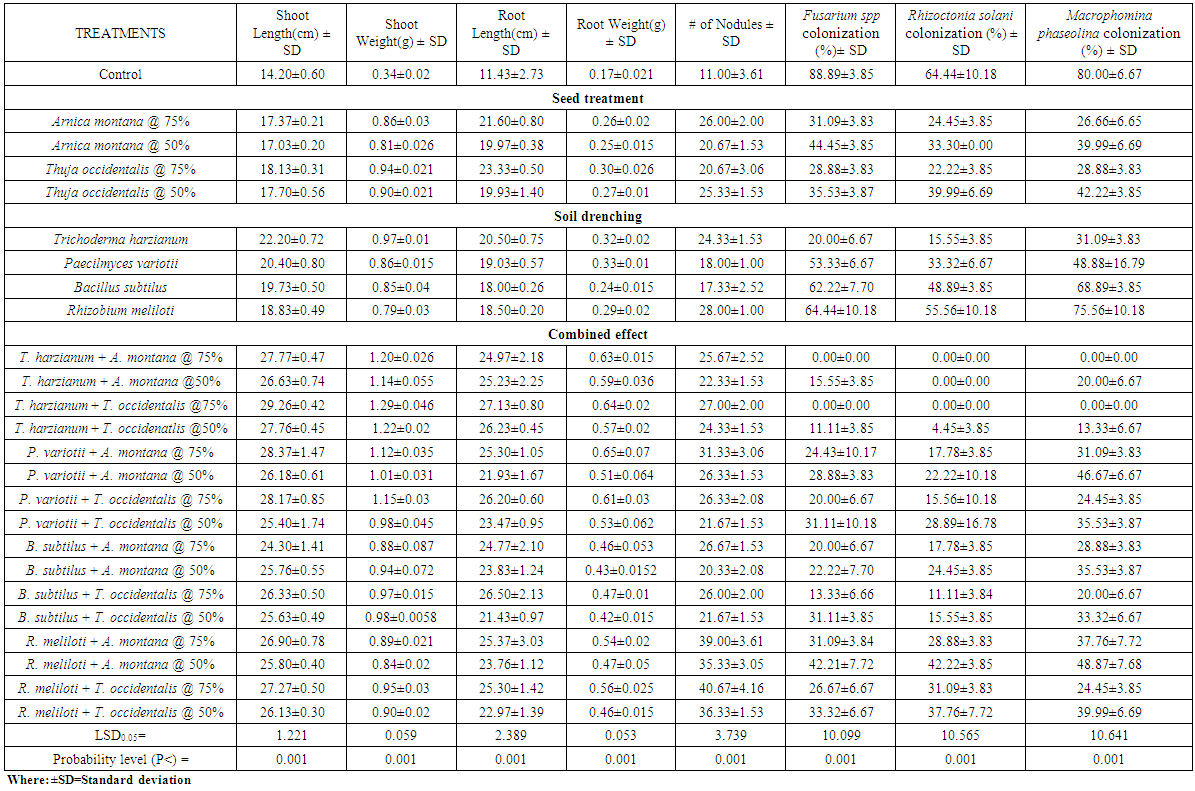
- Seeds of okra and sunflower treated with A. montana and T. occidentalis @ 50 and 75% v/v concentrations along with T. harzianum drenched in soil showed significant enhancement in the weight and height of plants but also completely suppressed Fusarium spp, Rhizoctonia solani and Macrophomina phaseolina colonization. When both concentrations of A. montana and T. occidentalis combined with P. variotii, B. subtilus and R. meliloti respectively, it increased the shoot and root weight. Maximum inhibition of root rot fungi was observed in all treatments except B. subtilus which showed complete inhibition of R. solani colonization when seeds treated with both homeopathic drugs @ 75% v/v concentration (Table 1.1 and 1.2). Whereas, when mung bean and mash bean seeds treated with T. occidentalis and A. montana @ 50 and 75% v/v concentrations and soil was drenched with Trichoderma harzianum, Paecilomyces variotii, Bacillus subtilus and Rhizobium meliloti improved the growth parameters. R. meliloti when used with treated seeds of A. montana and T. occidentalis @ 50 and 75% v/v concentrations showed greater number of nodules and increased root weight. Root rot fungi were completely inhibited by T. harzianum drenched soil along with seed treatment of both concentrations with homeopathic drugs. When soil was drenched with P. variotii, B. subtilus and R. meliloti and seeds treated with T. occidentalis and A. montana @ 50 and 75% v/v concentrations respectively showed maximum inhibition of Rhizoctonia solani, Fusarium spp and Macrophomina phaseolina colonization (Table 1.3 and 1.4).Results showed that seeds of sunflower, okra, mung bean and mash bean treated with A. montana and T. occidentalis @ 75% v/v concentration used along with microbial antagonists drenched in soil improved the plant growth followed by 50% v/v concentration respectively. However; off all the microbial antagonist, complete suppression of pathogenic fungi were observed in the roots of crop plants by T. harzianum drenched soil and seeds treated with both homeopathic drugs @ 75% v/v concentrations.
4. Discussion
- Seed treatment with T. occidentalis and A. montana (30C) and soil drenching with antagonistic organism showed significant results in controlling the root rot fungi and enhancement of plant growth. T. occidentalis contain an active ingredient such as mono terpene thujone used in the production of insecticides, pharmacologically, soaps, deodorants and perfumes (Kamden & Hanover, 1993). T. occidentalis extracts contain antiviral, antidiarrheal and sedative activities (Deb et al., 2007; Aziz et al., 2014). T. occidentalis (30 and 200M) inhibit A. flavus whereas 50M inhibit A. niger (Gupta & Srivastava, 2002). Arnica montana contain active constituents recognized in flower, leaves and roots having alcohols, flavonoids, sesquiterpene lactones, carotenoids, essential oil, tannins, phenolic and chlorogenic acids compounds (Gawlik-Dziki et al., 2011; Weremczuk-jezyna et al., 2011). A. montana exhibited antioxidant, antibacterial, antiseptic, antisclerotic and antifungal activities (Ganzera et al., 2008; Sugier & Gawlik-Dziki et al., 2009). A. montana (3, 6, and 12CH) improved the growth of plants (Bonfim et al., 2008). Hanif and Dawar (2015) reported that by using 100, 75 and 50% v/v concentrations of T. occidentalis and A. montana (30C) used as a seed treatment and soil drenching methods inhibit the root rot fungi and improved the growth of non leguminous and leguminous plants. A. montana and T. occidentalis (30C) pellets used in vitro and in vivo experiments showed positive results in suppressing root rot fungi (Hanif et al., 2015). Use of antagonistic bacteria applied as soil drenching or seed dressing showed significant inhibition of root rot pathogens on crop plants (Zaki & Ghaffar, 1987; Ehtesham-ul-Haque et al., 1990; Shahzad & Ghaffar, 1992). Besides fungal microbial antagonists, antagonistic bacteria also reduced the colonization of root rot fungi. Rhizobium spp., not only control soil borne pathogens but produce beneficial effect on plants by improving nutrient and water uptake of plants (Seuk Bae et al., 2000). Soil inoculated with the Bacillus cereus, Bacillus subtilus and Trichoderma spp. suppressed seedling infection and with the increase of dose it increase antagonist population (Bankole & Adebanjo, 1998). Paecilomyces produces peptidal antibiotic (P-168) which reduced root infecting fungi to a larger extent (Isogai et al., 1980). Soil application with Trichoderma viride and neem oil cake showed significant results in controlling the root knot nematode and Fusarium spp. in chick-pea which increased the yield (Pandey et al., 2005). Soil amended with A. javanica stem and leaves powder @ 1.0% w/w and seeds dressing with T. harzianum, P. aeruginosa and A. niger showed maximum plant growth and suppressed root rot fungi on cowpea and mung bean plants (Ikram & Dawar, 2015). Soil application and seed treatment with T. harzianum, T. viride and G. virens against R. bataticola found to be most effective in suppressing root rot disease but also improved germination, plant growth and yield of chick-pea (Dubey et al., 2011). Seed treatment with Bacillus subtilis and Gliocladium virens reduced the colonization of Fusarium spp in cotton (Zhang et al., 1996). Bio-priming of peanut, chickpea, okra and sunflower seeds with T. harzianum, R. meliloti and Bacillus sp. conidial/cell suspensions at five, ten and twenty minutes significantly suppressed Macrophomina phaseolina and Fusarium spp (Rafi & Dawar, 2014). Plant growth promoting Rhizobacteria improve plant growth through direct stimulation in the plant either producing growth regulators or suppressing pathogens (Raaijimaker et al., 2002; Weller et al., 2002). Seed treated with A. montana and T. occidentalis @ 75 and 50% v/v concentrations and soil drenching with T. harzianum showed excellent results in the control of root rot fungi followed by P. variotii, B. subtilus and R. meliloti respectively which was also reported by Abdel Kader et al. (2012) in which T. harzianum used alone or in combination significantly suppressed the soil borne pathogens as compared to Pseudomonas flourescens, Sacchromyces cerevisiae and Bacillus subtilis. Application of T. occidentalis (30C) as a seed treatment with 0.1% concentration increased the weight and length of shoot and root of cereal plants (wheat and millet) but also reduced the root infecting fungi (Dawar et al., 2015). Similar results were also obtained by Panda et al. (2013) and Baumgartner et al. (2004) which found that higher concentrations of drugs is stronger in action in case of pea plants. Homeopathic drugs used in very low doses and found to be environmental friendly and cheap (Toledo et al., 2011). Homoeopathic drugs fulfill the promise due to antifungal properties (Sinha & Singh, 1983; Shrivastava & Atri, 1998) and for that reason study on the homeopathic drugs needs to be enhanced (Benzie & Watchtel-Galor, 2011).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML