-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2015; 5(2): 29-37
doi:10.5923/j.ijmb.20150502.02
Assessment of Epiphytic Lichen Diversity in Pine Plantations and Adjacent Secondary Forest in Peacock Hill, Pussellawa, Sri Lanka
C. M. S. M. De Silva, S. P. Senanayake
Department of Botany, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka
Correspondence to: S. P. Senanayake, Department of Botany, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Lichens have been widely considered as sensitive bioindicators of forest health and ecological continuity as well as atmospheric pollution. This study was conducted to assess the variation of lichen diversity in pine plantations and adjacent secondary forest located in Peacock hill, Pussellawa in Central Province of Sri Lanka, and the potential of use those lichen species as bioindicators of environmental stress. The frequency of occurrence of lichen species on a defined portion of tree bark was used as an estimate of diversity and to evaluate the degree of environmental stress on the sensitive lichen community. In total 19 lichen taxa were recorded in trees in the study site of them 16 were crustose lichens, 03 foliose and no fruticose lichens were recorded. Lichen Diversity (LD) values were generated based on the recorded epiphytic lichens. Crustose lichen form was the most frequently encountered lichen form among study sites and the quality of the environment in pine plantations was assessed as relatively low compared to the adjacent secondary forest. This may also be due to the lack of lichen propagules or suitable substrata for colonization for lichens in the pine plantations. Lowest LD value of disturbed pine plantation, located in close proximity to the main road, and the highest LD value for secondary forest indicated the possible air pollution due to vehicular emissions, which could have influenced on the diversity of lichens. However, with respect to the previous researches, lichen diversity was very low in secondary forest compared to other pristine forests in Sri Lanka and that may be due to disruptions in ecological continuity that prevailing in the study site. Results of the present study suggest that lichens can be considered as potential indicators in assessing degree of environmental stress, air pollution and ecological continuity within different vegetations and in regenerating habitats.
Keywords: Lichen diversity, Environmental stress, Ecological continuity, Secondary forest, Pine plantation
Cite this paper: C. M. S. M. De Silva, S. P. Senanayake, Assessment of Epiphytic Lichen Diversity in Pine Plantations and Adjacent Secondary Forest in Peacock Hill, Pussellawa, Sri Lanka, International Journal of Modern Botany, Vol. 5 No. 2, 2015, pp. 29-37. doi: 10.5923/j.ijmb.20150502.02.
Article Outline
1. Introduction
- Lichen is an organism that is composed of a mycobiont (fungal partner) and a photobiont (alga or cyanobacteria) growing together in a symbiotic relationship, where the two organisms growing together rely on each other for their survival. The photobiont is sensitive to a wide range of environmental conditions which determine the distribution pattern of the lichen. The mycobiont contains a diversity of chemical substances for the protection of the photobiont which extend their ecological range. Thus lichens are sensitive to changes in atmospheric and micro-climatic conditions and have been used as bioindicators to determine the environmental stress in tropical and temperate countries [1]. Various factors govern the growth and development of lichens on tree trunks in different habitats. These include macroclimatic factors, microclimatic factors, site factors, substrate characteristics and etc [2-4]. Variation of macroclimatic factors; rainfall and temperature affect lichen development pattern in different geographical regions. Microclimatic factors such as light, humidity and temperature are the most vital factors that cause variation within the site. Site factors such as age, composition, management practices and pollution of the forest also affect lichen development. Substrate characteristics of tree species such as bark type, surface corrugation, moisture retention, pH and nutrient status of the bark influence the growth of lichens on a tree. But the influence of above factors on lichen development in a given ecosystem considerably varies [5]. A few recorded systematic studies on the distribution and diversity of lichens in different geographical regions are available in Sri Lanka due to the lack of knowledge of the taxonomy of tropical lichen species, their ecology and the community structure [6]. The first collection of lichens of Sri Lanka was made by G. H. K. Thwaites in 1868 followed by the recognition of 199 species by W.A. Leighton in 1870. Several researchers and authors [7-18] have contributed to increase the number of recorded lichen species in Sri Lanka [19]. Gunawardena and Wijeyaratna (2000) have revealed that significant changes have been caused in the existing lichen diversity in Ritigala forest due to microclimatic variations in different attitudes and also due to human activities that lead to severe atmospheric pollution. In 2010, Weerakoon et al. assessed the variation of lichen diversity in areas of different forest management practices under different environmental conditions in Dotalugala, Knuckles mountain range and found the significant variation in lichen diversity between disturbed and undisturbed vegetations. Studies of Attanayaka and Wijeyaratne (2013) clearly revealed that air pollution primarily caused due to vehicular emissions in highly disturbed city sites and in some suburban sites in the Western Province of Sri Lanka have an influence on the diversity and the distribution of lichens.At present, the extension of native forests has been reduced worldwide and their distribution is increasingly fragmented due to expanding of tree plantations and agricultural practices. The native forest located in Peacock hill has also been affected due to the expansion of tree plantations (Pinus caribaea and Eucalyptus sp.), tea estates and lands for other agricultural crops. Secondary forest in Peacock hill is regenerated largely through natural processes after significant removal and disturbance of the original forest vegetation by people over the time and display differences in forest structure and canopy species composition with compared to pristine forests. Lichen diversity and distribution in the Peacock hill have not been systematically documented or assessed so far. Hence the objective of the present study was to assess the variation of lichen diversity using lichen diversity values and to evaluate the prevailing environmental stress in disturbed and undisturbed pine plantations and, in the adjacent secondary forest located in Peacock hill, Pussellawa.
2. Materials and Methods
2.1. Collection Site and Sampling
- This study was carried out in Peacock hill located in Pussellawa in Sri Lanka (Figure 1). Geographical co-ordinates of Peacock hill are approximately 7° 05/ N and 80° 37/ E. Mean annual temperature is 22.9°C; mean annual precipitation lies between 2000-2500 mm. Pine plantations (disturbed and undisturbed) and a secondary forest were selected as study sites in Peacock hill and the study was carried out during the period of April to October 2013. Four sample plots (100 m x 100 m) were demarcated within each study sites using stratified sampling technique. The sample plots were selected in secondary forest based on accessibility and each plot was divided into four equal quadrats and one tree per quadrat was selected. Trees with girths more than 40 cm and showing no evidence of damage or interference by humans or animals were selected for sampling. The each sample plot was assigned under the two categories; i) plots in perimeter or ii) plots within the study site, based on the position of the plot.
 | Figure 1. Map of Sri Lanka indicating study site |
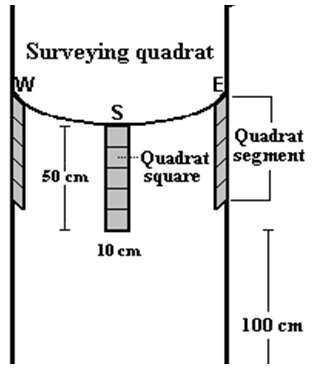 | Figure 2. Location of placing the quadrat on the tree trunk during the study |
2.2. Calculation of Lichen Diversity (LD) Values
- Thus European guideline developed by Asta et al. (2002a, b) was used to assess the lichen diversity in Peacock hill. The European Guideline has been recently applied in a number of major studies to map lichen diversity in temperate and tropical countries [24-27]. LD value for each sample plot was calculated following the procedures of Asta et al. (2002 a, b). Within each sample plot; a sum of frequencies of all lichen species at each aspect on each tree (1) was calculated. Thus for each tree there were four Sums of Frequencies (SF1) on the North (SF1N), East (SF1E) South (SF1S) and West (SF1W) side of the trunk. Then the Mean of the Sums of Frequencies (MSF) for each aspect (North, East, South, and West) in each sample plot (j) was calculated according to the following equation:MSFNj = (SF1Nj + SF2Nj + SF3Nj + SF4Nj) / nWhere;MSFNj = Mean of the sums of frequencies of all trees of sample plot j at a given aspect (e.g. North)SF1Nj = Sum of frequencies of all the species found at one aspect of tree 1 (e.g. North)N, E, S, W = north, east, south, westn = number of trees surveyed in sample plot jThe Lichen Diversity Value (LDV) of a sample plot was then calculated as the sum of the MSFs of all aspects:LDVj = (MSFNj + MSFEj + MSFSj + MSFWj)Where; LDVj = Lichen Diversity Value of sample plot j
2.3. Species Diversity
- Species diversity between study sites were determined using Sørensen coefficient (Ss) [28] according to the following equation:Ss = 2a ÷ (2a + b + c)Where, a is the number of common lichens in study site 1 and 2b is the total number of lichens in study site 1 c is the total number of lichens in study site 2
2.4. Collection of Lichens
- Foliose lichens were collected with part of the substrate to prevent any damages to the thallus and rhizines. Crustose species were cut off by taking sufficient bark from the trees. Ordinary brown paper bags were used as temporary pockets to collect lichen specimens.
2.5. Lichen Identification
- Morphological characters of thalli and fruiting bodies were examined using magnifying lenses (x10) and microscopic observations (Olympus microscope C X 21) of free hand sections were made wherever necessary to obtain characters to follow diagnostic keys. Chemical spot tests were carried out using freshly prepared Sodium hypochlorite solution and 10% aqueous solution of Potassium hydroxide. Lichen identification was carried out according to lichen identification keys and the pictorial guide “Lichens of tropical forests in Thailand: A field key to characteristic epiphytic species in northern Thailand” [1] and by comparing with authenticated specimens at the National Herbarium, Peradeniya, Sri Lanka.
3. Results
3.1. Epiphytic Lichen Distribution
- A total of 19 lichen taxa were recorded on trunks of 48 trees in the study sites of these 16 lichen species were crustose and 03 species were foliose. No fruticose lichens were observed in any of these sites. Crustose lichens are the simplest form of lichen. It is a crust on the surface. These type of lichens have lack or little organized thallus and are closely attached to the substratum. Foliose lichens are called as leafy lichens and the thallus is loosely attached to the substratum by rhizines or hapters with distinct upper and lower surfaces. Fruticose lichens are hair like, shrubby, finger like or strap shaped and attached to the substratum only at their bases and remaining major portion is either growing erect or hanging. Foliose lichens; Rimelia reticulata, Parmotrema sp., Heterodermia sp. and, crustose lichens; Lecanora orosthea, Pertusaria amara, Pertusaria sp., 02 Graphis sp., 03 Lepraria sp. and eight unidentified species were found in the study sites (Table 1).
|
 | Plate 1. Unidentified lichen spp |
3.2. Species Diversity
- According to the Sørensen coefficient, similarity of species diversity in disturbed and undisturbed pine plantations was found as 45.16%. The similarity of species composition in the undisturbed pine plantation and secondary forest was 25.81% and, in disturbed pine plantation and the secondary forest was 16.67%.
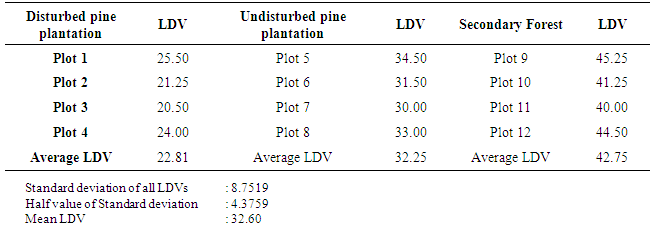
3.3. Lichen Diversity Values (LDV)
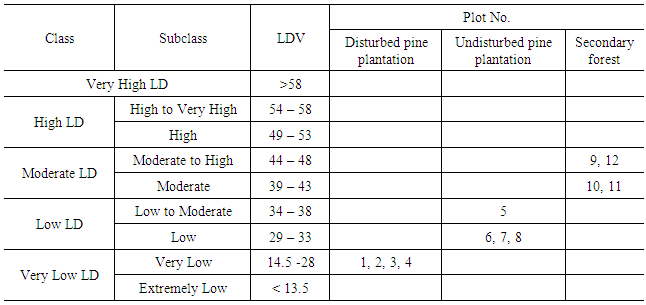
- The LDVs of plots were grouped into classes using the LD interpretation scale based on mean LDV and mean standard deviation of all LDV introduced by Asta et al. (2002 a,b) and Mulligan (2009). The lower limit of class 1 was zero.Interval of class 1 = mean LDV – 1/2 stdevWhere, stdev is the standard deviation and mean LDV is the mean Lichen Diversity ValueClass 2 = Class 1 + stdevClass 3 = Class 2 + stdevClass 4 = Class 3 + stdevClass 5 = > upper limit of Class 4The upper limit of class 5 was open.
|
3.4. Lichen Diversity Analysis
- The local scale distinguishes five LD classes and those were further divided into subclasses to provide a higher degree of resolution in lichen analysis. LD values of sample plots were assigned to the LD classes and LD subclasses as shown in Table 3.
|
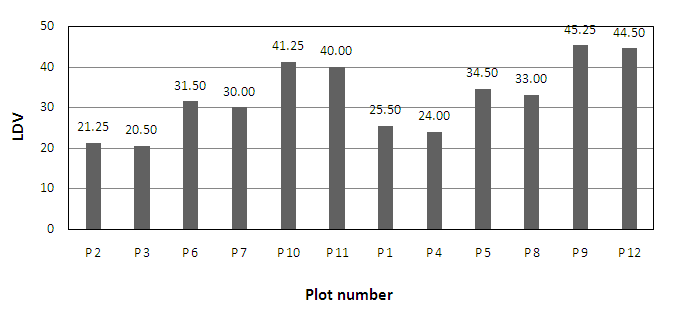 | Figure 3. LDVs in sampling plots in ‘perimeter' and 'within study site' |
3.5. Frequency on Tree Trunks Based on North, East, South, West Aspect of Trees
- According to the Figure 4, it was observed that greater frequency values were recorded on the trees in all aspects in the secondary forest than on the trees in pine plantations. In the secondary forest, the highest frequency was recorded on the eastern side of the trees (F=178). The highest frequency in undisturbed pine plantation was recorded on the southern side of the pine trees (F=141). In contrast to this the highest frequency for the disturbed pine plantation was recorded on the western side (F=98). The maximum lichen frequencies in the study sites in Peacock hill were on the south and west sides of the tree trunks with 25.43% and 25.05% of all frequencies (Figure 4). The lower frequency scores were recorded on trees with northern and eastern aspects with 24.66% and 24.86% of all frequencies respectively (Figure 5).
 | Figure 4. Frequency totals on aspects of tree trunks in three study sites |
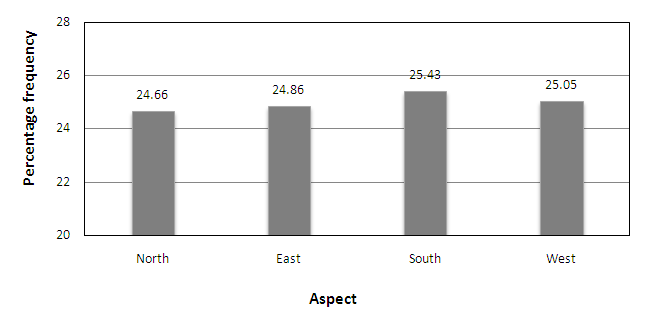 | Figure 5. The percentage frequencies of lichens on aspects of trees in study sites |
4. Discussion
- A greater number of crustose lichens were present in all the study sites whereas a few foliose lichens were present in undisturbed pine plantation and secondary forest. No fruticose lichens recorded on the trunks of trees in any of the study sites. The occurrence of fruticose and foliose lichens in canopies is related to higher precipitation in the canopy as well as relatively greater availability of light on twigs and branches. As Mulligan (2009) described, bark furrows found in pine trees are dark and dry and, are commonly inhabited by calicioid and leprose lichens. Thus leprose lichens were present in high numbers in both studied pine plantations. Few foliose lichens were recorded on tree trunks in the secondary forest (03) and, in undisturbed pine plantation (01) and none in disturbed pine plantation. This suggested that the secondary forest has relatively a lower disturbance level and high ecological continuity which supports the development of foliose lichens when compared with pine plantations.Results showed that 07 epiphytic lichen taxa occurring on pine trunks in disturbed pine plantation were crustose; including Graphis sp.1, Lepraria sp.3, unidentified sp.1, unidentified sp.2, unidentified sp.3, unidentified sp.4 and unidentified sp.6. The three most frequent (F) lichen species were unidentified sp.2 (F=155), unidentified sp.1 (F=83), unidentified sp.3 (F=47) (Table 1). As the plantation is located in the close proximity to the main road from Pussellawa to Doragala; pine trees are being exposed to vehicular emissions heavily and also the relative humidity in the site was low. Due to these prevailing disturbances in the site, no foliose and fruticose lichens were recorded on pine trunks in disturbed pine plantation. A total of 10 epiphytic lichen taxa were recorded in undisturbed pine plantation including 09 crustose lichens; Pertusaria amara, Graphis sp.1, Lepraria sp.1, Lepraria sp.3, unidentified sp.1, unidentified sp.2, unidentified sp.3, unidentified sp.4 and unidentified sp. 6 and 1 foliose lichen Parmotrema sp. The three most frequent (F) lichen species were unidentified sp.1 (F=168), unidentified sp.4 (F=103) and unidentified sp.2 (F=72) (Table 1). This site was located away from the main road (1km) and the mist is very frequent in the undisturbed pine plantation. Thus one foliose lichen species was recorded in the trunk but more foliose lichens were present in the canopy level. This may be related to the higher precipitation in the canopy as well as the relatively greater availability of light on twigs and branches [5]. Thirteen epiphytic lichen taxa including 10 crustose and 03 foliose were observed on trunks of trees in secondary forest. Crustose lichens include Lecanora orosthea, Pertusaria amara, Pertusaria sp., Graphis sp.1, Graphis sp.2, Lepraria sp.2, unidentified sp.5, unidentified sp.6, unidentified sp.7 and unidentified sp.8 while foliose lichen taxa were Rimelia reticulata, Parmotrema sp. and Heterodermia sp. The three most frequent (F) lichen species on tree trunks in secondary forest were, unidentified sp.6 (F=245), Pertusaria amara (F=90) and unidentified sp.5 (F=61) (Table 1). The number of foliose lichens available in the trunk level was comparatively higher due to the presence of high moisture that exists as mist throughout the forest vegetation as described by Mulligan (2009) and due to the lower level of disturbance that prevail in the secondary forest. Wolseley and Aguirre-Hudson in 1997 found that lichens with Trentepohlia as photobiont are more frequent in moist evergreen forests at all altitudes and areas where atmospheric humidity is high. According to the findings of Nayanakantha and Gajameragedara in 2003 Trentepohlia were present in moist environments in National Botanic Gardens, Peradeniya and Walkerawatta forest stand but the absence in the Kandy Municipal region indicated that Kandy Municipal region is rlatively a disturbed area. In the present study taxa with Trentepohlia were present only in shady and moist environments such as undisturbed pine plantation and secondary forest. Hence the restricted occurrence of Trentepohlioid taxa in disturbed pine plantation suggested the presence of environmental stress compared to other sites.The Sørensen coefficient indicated that lichen species present in both pine plantations was similar whereas different species were recorded in the secondary forest compared to the pine plantations. This shows the effects of differences in substrate characteristics of tree species such as bark type, surface corrugation, moisture retention, pH and nutrient status of the bark, as noted by James et al. (1977) and Mulligan (2009). Lichen diversityLichen diversity counts can be taken as estimates of environmental quality and stress that prevailing in the area, where high values correspond to good quality with low stress and low values indicate poor quality and high stress [22-23]. The overall pattern of the LD values in pine plantations demonstrated the clustering of values around the bottom of the scale and it indicates that the diversity of epiphytic lichens is low in pine plantations compared to secondary forest. This could be due to the absence of source of lichen propagules or suitable substrata for colonization for lichens when the natural vegetation has been removed and replaced by exotic species such as Pinus [20]. All LD values of plots in the disturbed pine plantation studied were fallen into ‘Very Low’ LD class and the subclass ‘Very-low’ (Table 3) confirming the disturbance prevailing in the pine plantation. LD values in undisturbed pine plantation were fallen into ‘Low’ class and LD values were more scattered around the subclass of ‘Low’ LD (Table 3). Thus disturbed pine plantation indicates that it has higher environmental stress compared to other sites as it disturbed by the main road due to vehicular emissions.The situation in the secondary forest was slightly different as all LD values fall into ‘Moderate LD’ class while they distributed equally in ‘Moderate’ and ‘Moderate to High’ subclasses (Table 3). The low level of disturbance and presence of moisture cause low level of environmental stress for epiphytic lichen development in the secondary forest. Highest diversity which was recorded for the secondary forest indicates that lichens may rapidly colonize on the available substrata, where fragments of undisturbed forest or large trees remain. When comparing with other researches; the lichen diversity was very low in secondary forest in Peacock hill compared to pristine forests that present in Sri Lanka. This may be due to the poor dispersal and slow recovery of specialist lichen species and absence of specialized habitats due to disrupting of ecological continuity. The higher LD values in sample plots at the study site perimeter (Figure 3) may be explained by the higher availability of light, which is one of the most important parameters for lichen species development [5]. But the difference between the mean LD values and mean species number between plots in perimeter and within study site were not significant (p>0.05). Because other influential factors including temperature, humidity, drying effect of wind and etc. also affect the development of lichen. But in previous findings Brodekova (2006) and Mulligan (2009) found that there is a significant difference between plots in perimeter and within study site. They have described light as one of the factors having a strong influence on the development of epiphytic lichens on trees in woodlands and in forest ecosystems. The lichen percentage frequency values among the study sites on aspect ranged from 24.66% to 25.43% (figure 4). It seems that in general, the lichens in Peacock hill were distributed evenly in different aspects of tree trunks viz. north, east, south and west.The findings of the present study agreed with the results and conclusions made out from the similar studies carried out in temperate and tropics [26-27, 30]. Further, the present results clearly demonstrated that lichens could be used as reliable indicators in assessing ecological continuity within different vegetations and in the colonization of the regenerating habitats.
5. Conclusions
- Based on the recorded epiphytic lichens and LD values calculated, the environmental quality of both pine plantations were assessed as relatively low. All LD values in disturbed pine plantation were fallen into ‘Very low LD’ class whereas LD values of undisturbed pine plantation were in the ‘Low LD’ class. LD values of secondary forest were fallen into ‘Moderate LD’ class indicating that the prevailing environmental stress is low compared to pine plantations. Considering the above facts the most influencing parameters for epiphytic lichen development at the trunk level in the study sites can be suggested as type of tree species, bark roughness, bark pH, nutrient availability, light, moisture, air quality, past woodland management practices and contemporary human impacts. Therefore the potential use of lichens as bioindicators to assess the degree of the disturbance and ecological continuity is highlighted from the present study. Highest diversity in the secondary forest indicates that lichens have the ability in rapid colonizing on the available substrata. However, the low lichen diversity in Peacock hill secondary forest compared to other pristine forests could be due to the poor dispersal potential and slow recovery of certain lichen species and absence of specialized microhabitats due to disrupting of ecological continuity. This preliminary research has provided a platform for future environmental monitoring studies based on lichen distribution. This would lead to perform large-scale systematic mappings in regular intervals to assess the changes in the abundance of epiphytic lichens caused by different environmental factors and to distinguish different type of polluted zones in Sri Lanka on the basis of the lichen populations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML