-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2015; 5(2): 23-28
doi:10.5923/j.ijmb.20150502.01
Arabidopsis thaliana Dynamic Phenotypic Plasticity in Response to Environmental Conditions
Azim M. Merchant, Karolina M. Pajerowska-Mukhtar
Department of Biology, University of Alabama at Birmingham, Birmingham, USA
Correspondence to: Karolina M. Pajerowska-Mukhtar, Department of Biology, University of Alabama at Birmingham, Birmingham, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Living organisms exhibit a nearly ubiquitous property of phenotypic plasticity that enables them to display a range of phenotypes under diverse environmental conditions, including both biotic and abiotic stresses. Phenotypic plasticity in plants can affect single cells, tissues, organs as well as the whole plant phenotypes including morphology, physiology and ecological relationships with other organisms. Leaf traits including shape and size are closely tied to photosynthetic capacity and thus are considered as important indicators for investigating plasticity. Here we describe a comprehensive study aimed to understand the roles of environmental conditions in shaping up the leaf morphology. We showed that wild type Arabidopsis thaliana leaves reduce their length and width when grown under environmentally suboptimal conditions. We also performed a comparative study of a loss-of-function mutant plants corresponding to eukaryotic GCN2 (general control nonderepressible 2) kinase. We demonstrated novel contributions of this universal regulatory factor in leaf architecture under stress-free and environmentally imposed stress conditions. Our data shed light on comprehending the underlying mechanisms of leaf shape plasticity in Arabidopsis thaliana.
Keywords: Arabidopsis thaliana, Phenotypic plasticity, Leaf morphology, GCN2
Cite this paper: Azim M. Merchant, Karolina M. Pajerowska-Mukhtar, Arabidopsis thaliana Dynamic Phenotypic Plasticity in Response to Environmental Conditions, International Journal of Modern Botany, Vol. 5 No. 2, 2015, pp. 23-28. doi: 10.5923/j.ijmb.20150502.01.
Article Outline
1. Introduction
- Plants, as sessile organisms, are constantly challenged with various biotic and abiotic stresses and phenotypic plasticity constitutes an effective way to help cope up with the environmental stresses [1-3]. This phenomenon, defined as the pattern of response to changes in environmental conditions, can affect many morphological traits in plants but is best manifested in leaf shape. Leaf size and shape is one of the most complex phenotypes of angiosperms that is tightly controlled by environmental and genetic factors, spatially and temporally coordinating cell expansion and cell cycle activity [4-6]. Environmentally controlled variations in leaf size and parameters related to it, such as length and width, have been well documented in a number of plant species [4, 6-10]. Arabidopsis thaliana (Arabidopsis), a dicot commonly used as a plant model system, has been nearly exclusively studied under controlled laboratory conditions, where light intensity, photoperiod, humidity and temperature are maintained constant throughout the life cycle of the plant [11, 12]. However, a limited number of studies were conducted over the last years that used field-grown Arabidopsis plants. Typically, these reports were focused on understanding the environmental influence on well-studied phenotypic responses, such as fruit number, germination, seed length and width, flowering time and flooding response [13-17]. A handful of studies published to date investigated the overall fitness of a specific Arabidopsis mutant grown under field conditions and addressed morphological changes likely resulting from varied environmental influences [18-20]. In addition, the reports on the use of transgenic Arabidopsis or mutant plants that specifically focus on the phenotypic plasticity of leaf shape as the function of environment are also limited. Eukaryotic GCN2 (general control nonderepressible 2) is a serine/threonine protein kinase that is involved in sensing starvation-induced stress affecting multiple cellular processes. GCN2 encodes a multidomain containing protein harboring histidyl-tRNA synthetase (HisRS) and kinase domain [21, 22]. In yeast and mammals, the uncharged tRNAs accumulate under amino acid starvation and bind with the HisRS domain. This, in turn, activates the kinase activity [23, 24] to trigger the downstream signaling pathway. Upon activation, the GCN2 phosphorylates α-subunit of eukaryotic initiation factor 2 (eIF2α) to derepress the translation of downstream target genes encoding transcription factors, i.e. activating transcription factor 4 (ATF4) and general control nonderepressible 4 (GCN4) in mammals and yeast, respectively [23, 25, 26]. A wide range of GCN2 functions in diverse eukaryotes has been already documented. This includes starvation sensing, growth and development, differentiation, immune responses and tumor cell survival [26]. Arabidopsis also possesses a conserved GCN2-eIF2α signaling pathway and it has been demonstrated that this cascade can be activated by a wide array of stimuli and stresses including wounding, phytohormones and herbicide treatment [27-30]. Recently, GCN2 has also been reported to function in the normal growth and development, including seed germination, chlorophyll accumulation and leaf shape [31]. While the roles of GCN2 in response to diverse stresses are emerging, the involvement of GCN2 in phenotypic plasticity remains unclear. Here, we report a comprehensive study describing the morphology of five most prominent rosette leaves of Arabidopsis wild type and GCN2-deficient mutant plants. The objectives of this study were to shed light on the extent of environmental influence on lengths and widths of individual leaves in a cruciferous rosette, and to understand the contribution of GCN2 gene to the plant’s ability to accurately execute these plastic responses. We discuss varied influence of the growth conditions on the leaf shape depending on the developmental stages of the plant. Our data provide novel insights into the involvement of a universal eukaryotic regulatory factor in shaping leaf length and width.
2. Materials and Methods
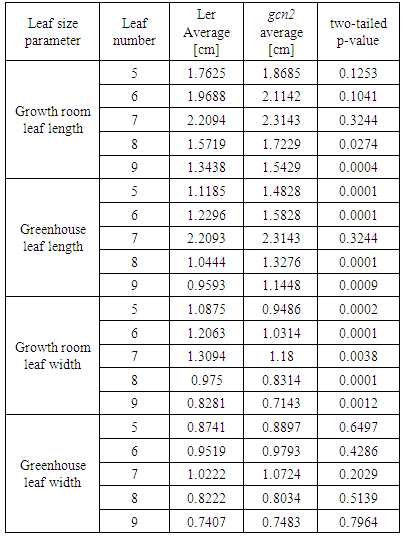
- Wild-type and gcn2 Arabidopsis plants used in this study are from the Landsberg erecta (Ler) ecotype. The Ler seeds were obtained from the Arabidopsis Biological Resource Center (ABRC; Ohio State University, OH, USA). The gcn2 Genetrap insertion line GT8359 was obtained from Cold Spring Harbor Laboratory, New York, USA (http://genetrap.cshl.org). The Genetrap lines carry a transposable element insertion (Ds) in Ler background [32]. Seeds were incubated for 72 h at 4°C to break dormancy and subsequently grown on MetroMix 360 (Sun Gro Horticulture) soil for 4 weeks. All plants were grown in 72-well flats and positions of the two genotypes were randomized. Two different types of growth conditions were used. The first set was grown in a tightly controlled growth room under 12 h light/12 h dark cycle at 21°C, 65% humidity and light intensity of 250 µmol m-2 sec-1. The second study was conducted during late spring/summer months (May and June) in the University of Alabama at Birmingham partially controlled greenhouse facility. Plants were grown under natural photoperiod (~14h light/10h dark) with temperatures varying between 15-25°C, humidity of 45-75% and light intensity ranging from 80-500 µmol m-2 sec-1 on overcast and sunny days, respectively. Once the plants reached to developmental stage # 3.50-3.70 (when rosette size reaches to 50%-70% of the final plant size) [33], leaves were numbered in chronological order, and leaves #5, 6, 7, 8 and 9 were harvested. Fifteen leaves per genotype per replicate were collected. Leaves were placed on moist paper towels to avoid dehydration and manual measurements of lengths and widths of the leaf blades were taken in triplicates. Data were subjected to statistical analyses (Student’s t-test) using SAS 9.3 software package (SAS Institute, Cary, NC).
3. Results and Discussion
3.1. Environmental Factors Affected the Dynamics of Rosette Area Development
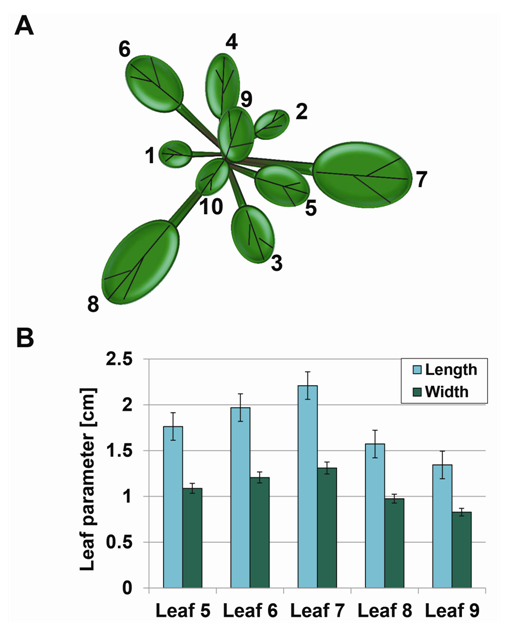
- Plants alter their morphology, physiology and cellular functions in response to varied environmental conditions. Leaf size and shape respond to changes in light levels and temperature and are important components of growth and development. We compared the leaf length and width as indicators of morphological plasticity under controlled growth room and greenhouse conditions. Four-week-old plants grown under either tightly controlled conditions in a growth room or partially controlled greenhouse conditions were at developmental stages # 3.50-3.70 (when rosette size is 50%-70% final size) [33]. Using the partly environmentally controlled greenhouse facility had a unique advantage in mimicking the field conditions for the following reasons. First, while the conditions were fluctuating depending on the weather and thus not optimal, they never fell out of the accepted range for Arabidopsis, thus ensuring a 100% plant survival and eliminating the possible adverse influence of sublethal temperature stress or presence of uncontrolled infectious agents. In addition, given that the local regulations don’t permit growing mutant or transgenic Arabidopsis in the field, using the greenhouse facility was the only feasible experimental system to be employed for our studies on the contribution of GCN2 to phenotypic plasticity.To quantify leaf size parameters, 16 plants per genotype per growth condition were subjected to systematic measurements of leaf length and width (Table 1). The length and width of Ler leaves #5–9 grown under stress-free conditions range from an average of 1.34 to 2.20 cm and 0.82 to 1.31 cm, respectively (Figure 1A, 1B).
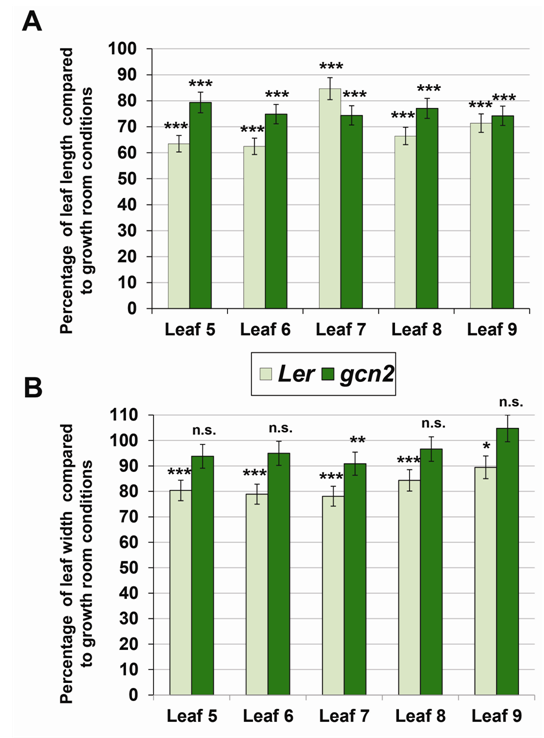 | Figure 2. A. Percentage of leaf length reduction in greenhouse-grown Ler and gcn2 plants compared to growth room-grown counterparts. Error bars represent standard error. Experiment was performed in triplicate with similar results. *** - p<0.0001, Student’s t-test. B. Percentage of leaf width reduction in greenhouse-grown Ler and gcn2 plants compared to growth room-grown counterparts. Error bars represent standard error. Experiment was performed in triplicate with similar results. n.s. – p>0.05, * - p<0.05, ** - p<0.001, *** - p<0.0001, Student’s t-test |
3.2. Influence of AtGCN2 on Leaf Phenotypic Plasticity
- GCN2 is a global regulatory factor that is involved in controlling evolutionarily conserved signal transduction pathways responsible for sensing starvation [24]. We grew a loss-of-function gcn2 mutant under growth room and greenhouse conditions and compared leaf growth of leaves # 5 through 9 to wild type Ler plants. In the overall comparison between the gcn2 mutant plants grown under two contrasting conditions, we observed that plants grown in the greenhouse display a smaller leaf size compared to the growth room conditions. These findings are in agreement with the overall reduction of leaf length and leaf width determined for Ler plants. However, the measurements of the individual gcn2 leaves generated certain intriguing results as summarized in Figure 2A and 2B and Table 2. The percent reduction of length for the leaves of gcn2 mutant grown under greenhouse conditions ranges between 74 and 79%. While statistically highly significant for all the leaves under investigation, this length reduction for gcn2 leaves is of a lesser extent compared to the percent length reduction in the corresponding Ler leaves (Figure 2A).
|
3.3. Contribution of GCN2 in Overall Plant Fitness
- To gain a deeper understanding of the GCN2 contributions to complex leaf shape phenotypes under varied environmental conditions, we next calculated the ratios between leaf lengths and widths between greenhouse and growth room conditions, which is a widely accepted way to study the leaf size [31]. To obtain a comparative measure of the differences between the two genotypes tested while including the varied input of growth conditions, we calculated ratios of leaf length and leaf width between the individual leaves of gcn2 and Ler grown under growth room and greenhouse conditions (Figure 3; example formula below).
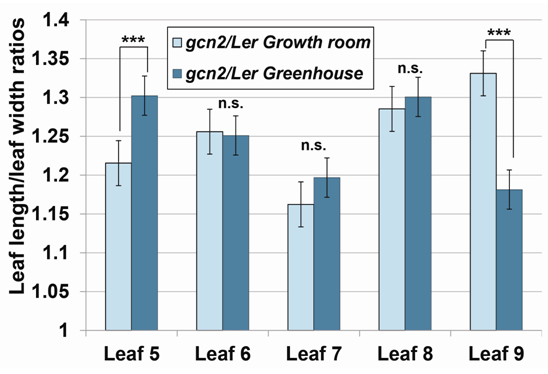 | Figure 3. Ratios of leaf length and leaf width between the individual leaves of gcn2 and Ler grown under growth room and greenhouse conditions. n.s. – p>0.05, *** - p<0.0001, Student’s t-test |
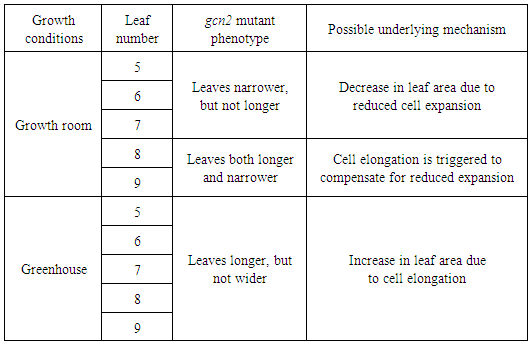
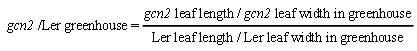 Overall, we concluded that the variable environment of greenhouse had adverse effects on leaf sizes in both genotypes tested, but was manifested differentially for each individual leaf and more profound in the Ler plants compared to the gcn2 mutants. Moreover, we determined that leaves # 5 and 9 can specifically serve as markers to capture the influence of GCN2 on leaf phenotypic plasticity. This discovery will allow for more targeted analyses in the future.The regulation of leaf size in Arabidopsis is still only partially understood, and proposed to rely on a number of processes including a complex spatial and temporal coordination of cell expansion and cell cycle activity [36, 37]. Any perturbation within these processes, such as loss of function of a specific gene, might translate into altered size and shape of a leaf. In general, smaller leaves are produced as a result of decreased cell size, resulting from reduced expansion and elongation, or a combination of both factors [36, 37]. Recently, we described the roles of AtGCN2 in plant hormone gibberellic acid (GA)-mediated regulation of germination and chlorophyll content [31]. The phenotypes of the gcn2 plants are reminiscent of plants deficient in GA biosynthesis or signaling. It has been previously shown that GA can control leaf size and shape through regulation of cell division and cell expansion [38]. For example, ectopic overexpression of GA 20-oxidase 1 (GA20OX1), which catalyzes important steps in GA synthesis, causes an enlargement of younger leaves when ectopically expressed in Arabidopsis [39, 40]. GAs have been also implicated in the control of cell proliferation. In the quadruple DELLA mutant, in which these growth repressors in GA signaling are down-regulated, cell proliferation and cell expansion rates were shown to increase [41]. In addition, a handful of reports indicate a role of GAs in cell elongation in various plant systems [42-44]. Consistent with this evidence, our data illustrate that in the growth room-grown gcn2 plants, the youngest leaves (# 8 and 9) are both narrower and longer (Table 2), indicating that strongest impact of decreased GA signaling. In contrast, and further corroborating the age-specific role of GA in foliar morphology, the older gcn2 leaves (# 5, 6 and 7) showed only effects on the leaf blade width (Table 2). The role of GA in phenotypic plasticity has been proposed previously [45, 46] and our study lends additional insights into the possible links to this important phytohormone in shaping plant environmental responses.
Overall, we concluded that the variable environment of greenhouse had adverse effects on leaf sizes in both genotypes tested, but was manifested differentially for each individual leaf and more profound in the Ler plants compared to the gcn2 mutants. Moreover, we determined that leaves # 5 and 9 can specifically serve as markers to capture the influence of GCN2 on leaf phenotypic plasticity. This discovery will allow for more targeted analyses in the future.The regulation of leaf size in Arabidopsis is still only partially understood, and proposed to rely on a number of processes including a complex spatial and temporal coordination of cell expansion and cell cycle activity [36, 37]. Any perturbation within these processes, such as loss of function of a specific gene, might translate into altered size and shape of a leaf. In general, smaller leaves are produced as a result of decreased cell size, resulting from reduced expansion and elongation, or a combination of both factors [36, 37]. Recently, we described the roles of AtGCN2 in plant hormone gibberellic acid (GA)-mediated regulation of germination and chlorophyll content [31]. The phenotypes of the gcn2 plants are reminiscent of plants deficient in GA biosynthesis or signaling. It has been previously shown that GA can control leaf size and shape through regulation of cell division and cell expansion [38]. For example, ectopic overexpression of GA 20-oxidase 1 (GA20OX1), which catalyzes important steps in GA synthesis, causes an enlargement of younger leaves when ectopically expressed in Arabidopsis [39, 40]. GAs have been also implicated in the control of cell proliferation. In the quadruple DELLA mutant, in which these growth repressors in GA signaling are down-regulated, cell proliferation and cell expansion rates were shown to increase [41]. In addition, a handful of reports indicate a role of GAs in cell elongation in various plant systems [42-44]. Consistent with this evidence, our data illustrate that in the growth room-grown gcn2 plants, the youngest leaves (# 8 and 9) are both narrower and longer (Table 2), indicating that strongest impact of decreased GA signaling. In contrast, and further corroborating the age-specific role of GA in foliar morphology, the older gcn2 leaves (# 5, 6 and 7) showed only effects on the leaf blade width (Table 2). The role of GA in phenotypic plasticity has been proposed previously [45, 46] and our study lends additional insights into the possible links to this important phytohormone in shaping plant environmental responses.4. Conclusions
- Overall, our results demonstrate an important function of GCN2 kinase in controlling phenotypic plasticity of global leaf shape under varied environmental conditions, which may be accomplished by influencing GA biosynthesis or signaling in Arabidopsis.
ACKNOWLEDGEMENTS
- The authors wish to thank Ms. Xiaoyu Liu and Ms. Kristin Rockett for technical assistance. This work was supported by NSF-CAREER award (IOS-1350244) to KPM.
References
| [1] | de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytol 2005;166:73-82. |
| [2] | Gratani L. Plant Phenotypic Plasticity in Response to Environmental Factors. Advances in Botany 2014. |
| [3] | Matesanz S, Gianoli E, Valladares F. Global change and the evolution of phenotypic plasticity in plants. Annals of the New York Academy of Sciences 2010;1206:35-55. |
| [4] | McClendon JH, McMillen GG. The control of leaf morphology and the tolerance of shade by woody plants. Botanical Gazette 1982;143:79–83. |
| [5] | Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in plant science 2000; 5:537-42. |
| [6] | Tsukaya H. Leaf shape: genetic controls and environmental factors. The International journal of developmental biology 2005; 49:547-55. |
| [7] | Dengler NG. Comparative histological basis of sun and shade leaf dimorphism in Helianthus annuus. Canadian Journal of Botany 1980;58:717–30. |
| [8] | Kitao M, Lei TT, Koike T, Tobita H, Maruyama Y. Susceptibility to photoinhibition of three deciduous broadleaf tree species with different successional traits raised under various light regimes. Plant, Cell and Environment 2000;23:81–9. |
| [9] | Markesteijn L, Poorter L, Bongers F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. American journal of botany 2007;94:515-25. |
| [10] | Wyka TP, Oleksyn J, Zytkowiak R, Karolewski P, Jagodzinski AM, Reich PB. Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: a common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 2012;170:11-24. |
| [11] | Koornneef M, Meinke D. The development of Arabidopsis as a model plant. The Plant journal: for cell and molecular biology 2010;61:909-21. |
| [12] | Meyerowitz EM. Arabidopsis thaliana. Annual review of genetics 1987;21:93-111. |
| [13] | Malmberg RL, Held S, Waits A, Mauricio R. Epistasis for fitness-related quantitative traits in Arabidopsis thaliana grown in the field and in the greenhouse. Genetics 2005;171:2013-27. |
| [14] | Mishra Y, Jankanpaa HJ, Kiss AZ, Funk C, Schroder WP, Jansson S. Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC plant biology 2012;12:6. |
| [15] | Moharekar Lokhande S, Moharekar S, Kobayashi T, Ishii H, Sumida A, Hara T. Phenotypic plasticity and ecotypic variations in growth and flowering time of Arabidopsis thaliana (L.) under different light and temperature conditions. Indian journal of experimental biology 2014;52:344-51. |
| [16] | Pigliucci M, Kolodynska A. Phenotypic plasticity to light intensity in Arabidopsis thaliana: invariance of reaction norms and phenotypic integration. Evol Ecol 2002;16:27-47. |
| [17] | Pigliucci M, Kolodynska A. Phenotypic plasticity and integration in response to flooded conditions in natural accessions of Arabidopsis thaliana (L.) Heynh (Brassicaceae). Annals of botany 2002;90:199-207. |
| [18] | Heidel AJ, Clarke JD, Antonovics J, Dong X. Fitness costs of mutations affecting the systemic acquired resistance pathway in Arabidopsis thaliana. Genetics 2004;168:2197-206. |
| [19] | Heidel AJ, Dong X. Fitness benefits of systemic acquired resistance during Hyaloperonospora parasitica infection in Arabidopsis thaliana. Genetics 2006;173:1621-8. |
| [20] | Wagner R, Aigner H, Pruzinska A, Jankanpaa HJ, Jansson S, Funk C. Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol 2011;191:449-58. |
| [21] | Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics 2000;154:787-801. |
| [22] | Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Molecular and cellular biology 1995;15:4497-506. |
| [23] | Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annual review of microbiology 2005;59:407-50. |
| [24] | Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochemical Society transactions 2006;34:7-11. |
| [25] | Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America 2004;101:11269-74. |
| [26] | Murguia JR, Serrano R. New functions of protein kinase Gcn2 in yeast and mammals. IUBMB life 2012;64:971-4. |
| [27] | Lageix S, Lanet E, Pouch-Pelissier MN, Espagnol MC, Robaglia C, Deragon JM, et al. Arabidopsis eIF2alpha kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC plant biology 2008;8:134. |
| [28] | Faus I, Zabalza A, Santiago J, Nebauer SG, Royuela M, Serrano R, et al. Protein kinase GCN2 mediates responses to glyphosate in Arabidopsis. BMC plant biology 2015;15:14. |
| [29] | Li MW, AuYeung WK, Lam HM. The GCN2 homologue in Arabidopsis thaliana interacts with uncharged tRNA and uses Arabidopsis eIF2alpha molecules as direct substrates. Plant biology 2013;15:13-8. |
| [30] | Zhang Y, Wang Y, Kanyuka K, Parry MA, Powers SJ, Halford NG. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2alpha in Arabidopsis. Journal of experimental botany 2008;59:3131-41. |
| [31] | Liu X, Merchant A, Rockett KS, McCormack M, Pajerowska-Mukhtar KM. Characterization of Arabidopsis thaliana GCN2 kinase roles in seed germination and plant development. Plant signaling & behavior 2015;10:e992264. |
| [32] | Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, et al. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes & Development 1995;9:1797-810. |
| [33] | Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, et al. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant cell 2001;13:1499-510. |
| [34] | Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH. Adaptive phenotypic plasticity: consensus and controversy. Trends in Ecology & Evolution 1995;10:212-7. |
| [35] | Dorn LA, Pyle EH, Schmitt J. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution; international journal of organic evolution 2000;54:1982-94. |
| [36] | Beemster GT, Fiorani F, Inze D. Cell cycle: the key to plant growth control? Trends in plant science 2003;8:154-8. |
| [37] | Gonzalez N, De Bodt S, Sulpice R, Jikumaru Y, Chae E, Dhondt S, et al. Increased leaf size: different means to an end. Plant physiology 2010;153:1261-79. |
| [38] | Hooley R. Gibberellins: perception, transduction and responses. Plant molecular biology 1994;26:1529-55. |
| [39] | Coles JP, Phillips AL, Croker SJ, Garcia-Lepe R, Lewis MJ, Hedden P. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. The Plant journal : for cell and molecular biology 1999;17:547-56. |
| [40] | Huang S, Raman AS, Ream JE, Fujiwara H, Cerny RE, Brown SM. Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant physiology 1998;118:773-81. |
| [41] | Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, et al. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current biology : CB 2009;19:1188-93. |
| [42] | Keyes G, Sorrells ME, Setter TL. Gibberellic Acid Regulates Cell Wall Extensibility in Wheat (Triticum aestivum L.). Plant physiology 1990;92:242-5. |
| [43] | Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, et al. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proceedings of the National Academy of Sciences of the United States of America 2013;110:4834-9. |
| [44] | Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, et al. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Current biology 2012;22:1183-7. |
| [45] | Kurepin LV, Mancell L, Reid DM, Pharis RP, Chinnappa CC. Possible roles for ethylene and gibberellin in the phenotypic plasticity of an alpine population of Stellaria longipes. Canadian Journal of Botany 2006;84:1101-9. |
| [46] | Pigliucci M, Schmitt J. Phenotypic plasticity in response to foliar and neutral shade in gibberellin mutants of Arabidopsis thaliana. Evolutionary Ecology Research 2004;6:243–59. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML