Sumita Sen1, Margaret E. Smith1, Timothy L. Setter2
1Department of Plant Breeding and Genetics, Cornell University, Ithaca, USA
2Department of Crop and Soil Science, Cornell University, Ithaca, USA
Correspondence to: Sumita Sen, Department of Plant Breeding and Genetics, Cornell University, Ithaca, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Nitrogen deficiency causes significant reduction in yield in maize. The objectives of present study was to evaluate the performance of various shoot traits in selected maize genotypes for tolerance to low nitrogen stress and to understand the morphological changes that take place in the plants under nitrogen stress condition. The intermated B73-Mo17 (IBM) recombinant inbred lines were used, because the parents differ in response to nitrogen stress while the recombinant inbred lines have been thoroughly genotyped. Ten IBM genotypes from the published Maize Genome Database database were evaluated in this study along with their parents B73 and Mo17. Plants were grown in Guterman Green House of Cornell University, USA. Two nitrogen treatments (solution with high N contained 65.79 g Ca (NO3)2 4H2O in 100L of 1X solution making 5.0mM NO3 and solution with low N contained 2.63 g Ca (NO3)2 4H2O in 100L of 1X solution making 0.2 mM NO3) were given to understand the effect of different nitrogen levels. Genotypes were a significant source of variation for all leaf traits except leaf width. Genotype by nitrogen treatment interaction was significant for shoot length and stalk length. Most of the IBM RILs performed better than the parents in terms of stalk length at low N. Parent B73 had longer leaf length (at low N) and shoot length (at both N levels) than Mo17, indicating that in B73, photoassimilates were transferred towards the development of leaves rather than towards stalk elongation (stalk length was similar for B73 and Mo17 at low N). IBM284 had the highest shoot length under both low and high nitrogen conditions.IBM337 had relatively long stems at low nitrogen but responded very little to additional N and thus had the shortest stems at high N compared to other genotypes. The parents B73 and Mo17 had positive effect on inheritance of stress tolerance as genotypes with high B73 composition performed as good as the parent B73 for all shoot traits except the leaf width.
Keywords:
IBM population, Low nitrogen stress, Nitrogen use efficiency, Shoot growth, Zea mays L.
Cite this paper: Sumita Sen, Margaret E. Smith, Timothy L. Setter, Effect of Low Nitrogen Stress on Various Shoot Traits of Maize (Zea mays L.), International Journal of Modern Botany, Vol. 5 No. 1, 2015, pp. 18-22. doi: 10.5923/j.ijmb.20150501.03.
1. Introduction
Maize is one of the most important cereal crops globally and ranked first in seed yield production in the world [1]. Nitrogen deficiency causes significant reduction in yield in maize. Plant vigor in terms of shoot growth is also affected severely. The common effects of nitrogen deficiency on maize plants are in relative male and female flowering time (anthesis-silking interval), number of ears per plant, and number of kernels per ear [2]. The environmental and economic issues limit excessive nitrogen fertilizer use and here lies the importance of developing new varieties with higher nitrogen use efficiency (NUE) [3].In case of cereals, NUE which is a quantitative trait controlled by many genes, is defined as the grain produced per unit of N used. Maize NUE varies from 25-50% [4] which indicates that most of the N applied is lost to the environment [5]. Developing hybrids with high NUE can prove to be the best option for increasing the grain yield under low N conditions and also maintaining the health of the environment.The Intermated B73 X Mo17 (IBM) population is an Advanced Intercross Line maize population developed by including four generations of random mating following the formation of the F2 generation of B73 x Mo17 and before the development of inbred lines [6]. The maize F1 hybrid produced by the crossing of B73 and Mo17 is taller, matures earlier and produces higher yield than either of the parents [7]. Identification of nitrogen tolerant lines will help in the development of further improved hybrids that can minimize yield losses and retain grain protein levels on soils with limited available N [8]. In the present study, we attempted to pre-select IBM families based on their genetic constitution at five target regions identified in previous QTL studies [9] associated with NUE and understand the effect of low and high nitrogen on various shoot traits, and thus identify inbreds with better NUE.
2. Materials and Methods
2.1. Plant Material
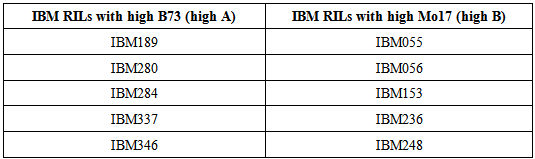
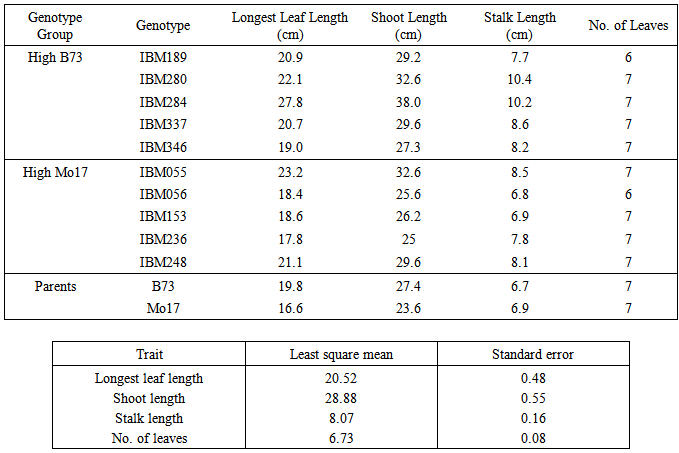
A list of 260 IBM Recombinant Inbred Lines (RILs) was obtained from the Maize Genome Database (Maize GDB, 2004) (http://maizegdb.org/). The RILs were classified into two groups based on the constitution of genotype (abundance of B73 and Mo17) [9] and nitrogen use efficiency (NUE). The IBM genotypes which have more of B73 in the five target regions are classified as IBM RILs with high B73. The IBM genotypes which have more of Mo17 in the regions are classified as IBM RILs with high Mo17. Five genotypes from each group (high B73 and high Mo17) were selected for the experiment along with parental inbreds B73 and Mo17 to compare the difference among the genotypes and the parents (Table 1).Table 1. List of IBM RILs selected for screening for nitrogen use efficiency
 |
| |
|
2.2. Growth Conditions
The ten IBM RILs and their parents (B73 and Mo17) were planted in the greenhouse. A total of 120 plants were grown in black plastic cylindrical pots. Sand was used as growth media for the maize seedlings. Fine, medium and coarse sand were mixed well and used to fill the 120 pots to equal depth. Two seeds were sown in each pot and seedlings were thinned to one per pot shortly after germination.Day temperature was about 70ºF and night temperature 60ºF. Along with normal sunlight, artificial lights (1000 Watt metal halide lamps) were provided to supplement enough light for plant growth.
2.3. Preparation of Nutrient Solution and Application
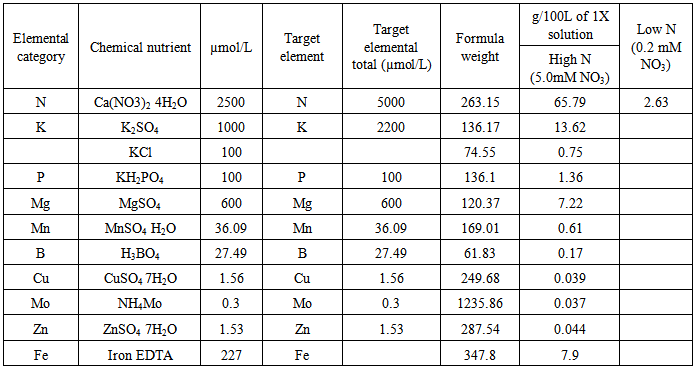
Nutrient solutions were prepared following Engels and Kirby (2001) [10]. Three stock solutions were prepared: solution A in high and low N versions and solution B. Solution A comprised 10L of high N and 10L of low N prepared in two different plastic containers (Table 2). Iron-EDTA was added in solution A. Solution B contained all other nutrients except Ca(NO3)2 4H2O and Iron-EDTA and a total of 20L was prepared in a plastic container. The stock solution was diluted to 10X solution. Beginning at eight days after planting, 30ml solution was applied to each pot and watering was done every other day. At one month after sowing, 30ml solution was applied every day and continued for 20 days. The plants were harvested at 50 days after planting. Then observations related to plant morphology were taken. Directions for preparing 10X stock solutions1. Add Iron-EDTA and Ca (NO3)2 4H2O as per Table 2 in 10L water. Label as solution A high N or solution A low N.2. Add all other chemical nutrients to 10L water in a separate container and mix well. Label as solution B.3. To mix up X liters of final solution, add 0.1X of either high or low N version of solution A to about 0.7X liters of water, mix well, and then add 0.1X liters of solution B. Mix and add water to final volume of X liters.Table 2. Nutrient solution composition
 |
| |
|
Solution applicationBeginning at eight days after planting, 30ml solution was applied to each pot and watering was done every other day. At one month after sowing, 30ml solution was applied every day and continued for 20 days.
2.4. Measurements of Shoot Traits
Leaf width of the longest leaf was taken. Approximately the middle portion of the leaf was chosen for the leaf width measurement. Number of leaves was determined by counting the leaves from the base to the tip of the plant. The shoot length of the plants was measured from the base of the shoot to the longest leaf tip. The stalk length of the plant was measured from the base of the shoot to the end point from where the last leaf emerged. Length of the longest leaf of each plant was measured from the base of the shoot to the longest leaf tip.
2.5. Experimental Design and Statistics
A split plot design was used for the experiment with five replications. Statistical analysis of various observations was done using the JMP software package.
3. Results and Discussion
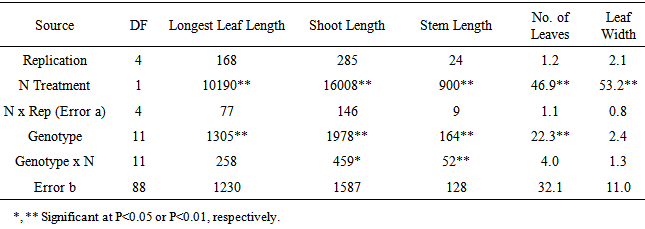
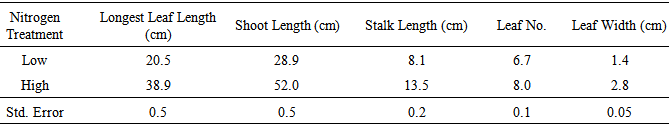
Nitrogen treatments significantly affected all the shoot variables measured. High N increased leaf length, leaf width and number of leaves. Parent B73 had longer shoots and leaves under low N conditions compared to parent Mo17. The IBM RILs with high B73 composition in those genomic regions with QTLs for root- and nitrogen-related traits also had longer shoots and leaves, as well as longer stalks, under low N conditions compared to IBM RILs with high Mo17. However, the IBM RILs with high Mo17 had wider leaves than the high B73 RILs. N treatment X genotype interaction was significant for stalk length and shoot length. Table 3. Sums of Squares from Analysis of Variance for Shoot Traits
 |
| |
|
Table 4. Mean values for shoot traits at low and high nitrogen levels, evaluated for 12 genotypes in five replications in the greenhouse
 |
| |
|
Table 5. Mean values of different shoot traits of 12 maize genotypes grown under low nitrogen condition in five replications evaluated in green house
 |
| |
|
3.1. Stalk Length
Between the two parent inbreds, stalk length was similar at low N but Mo17 responded more (i.e., had longer stalks) at high N than did B73. Most IBM RILs had longer stalks than either parent at low N, while IBM284 and IBM280 (both high B73 RILs) had longer stalks than either parent and than any of the other RILs at both N levels. Genotype IBM337 had relatively long stalks at low nitrogen but responded very little to additional N and thus had the shortest stalks at high N. Genotypes IBM056 and IBM153 had longer stalks than either parent at both high and low nitrogen levels.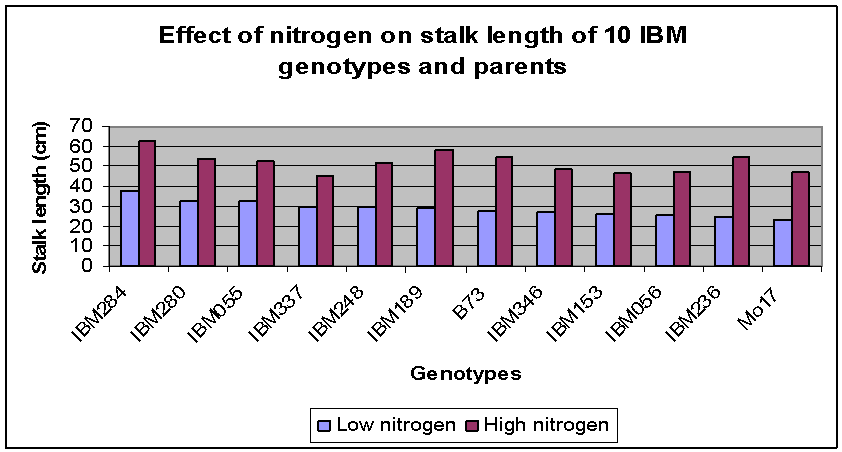 | Figure 1. Effect of nitrogen treatment on stalk length of 12 maize genotypes evaluated in five replications in the greenhouse |
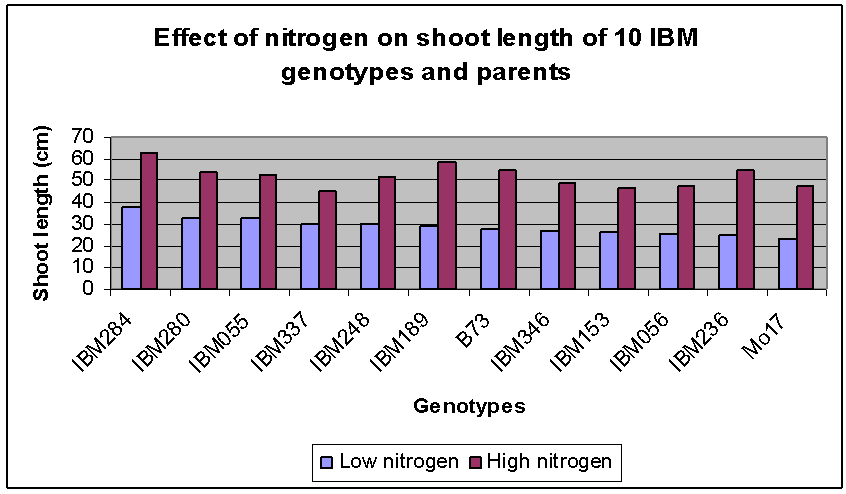 | Figure 2. Effect of nitrogen treatment on shoot length of 12 maize genotypes evaluated in five replications in the greenhouse |
3.2. Shoot Length
For shoot length, performance of the parents at high N was reversed compared to that noted for stalk length (i.e., B73 had longer shoots at high N than did Mo17). This same relationship was maintained at low N. IBM236 showed to have almost same shoot length as the parent Mo17 under high N condition. IBM284 had the highest shoot length under both low and high N conditions. Significance of the shoot treatment values are checked against the least square mean and standard error values.Parent B73 had longer leaf length (at low N) and shoot length (at both N levels) than Mo17, indicating that in B73, photoassimilates were transferred towards the development of leaves rather than towards stem elongation (stalk length was similar for B73 and Mo17 at low N).In understanding of the effects of N treatments on shoot traits and the association between shoot traits and NUE may be important in determining the appropriate selection criteria and breeding techniques under low-N conditions.
4. Conclusions
In general, some genotypes performed about the same in terms of shoot length but on average genotypes with high B73 composition had better performance than the genotypes with high Mo17 composition. In the future, a better understanding of the genetic basis for maize growth and development, adaptiveness and grain production under low N conditions is required to improve the selection efficiency for such environments in both developing and developed countries.
ACKNOWLEDGEMENTS
The authors would like to thank Guterman Laboratory members of Cornell University for their technical assistance.
References
| [1] | Stephen, P.L., Zhu, X.G., Naidu, S.L., and Donald R., 2006, Can improvement in photosynthesis increase crop yield. Plant Cell and Environment, 29, 315-330. |
| [2] | Ribaut, J.M., Fracheboud, Y., Monneveux, P., Banzinger, M., Vargas, M., and Jiang C., 2007, Quantitative trait loci for yield and correlated traits under high and low soil nitrogen conditions in tropical maize. Molecular Breeding, 20, 15 – 29. |
| [3] | Geiger, H.H., 2009, Agronomic Traits and Maize Modifications: Nitrogen Use Efficiency. “Handbook of Maize: Its Biology”, pp405-417. J.L. Bennetzen, S.C. Hake eds. Springer Science and Business Media, LLC. |
| [4] | Tilman, D., Cassman, K.G., Matson, P.A., Naylor, R., and Polasky, S., 2002, Agricultural sustainability and intensive production practices. Nature, 418, 671–677. |
| [5] | Moose, S., and Below, F.E., 2009, Biotechnology approaches to improving maize nitrogen use efficiency. “Molecular genetic approaches to maize improvement”. pp65-77. Vol 63. Part II. Kriz, A.L., and Larkins, B.A., (Eds). Springer Berlin Heidelberg. |
| [6] | Lee, M., Sharopov, N., Beavis, W.D., Grant, D., Katt, M., Blair, D., and Hallauer, A., 2002, Expanding the genetic map of maize with the intermated B73 × Mo17 (IBM) population. Plant Molecular Biology, 48, 453–461. |
| [7] | Hallauer, A.R., and Miranda, J.B., 1981, Quantitative Genetics in Maize Breeding. Iowa State Univ. Press, Ames. |
| [8] | Massey, P., and Warsi, M.Z.K., 2009, Influence of low nitrogen and excess soil moisture stress on yield of maize inbreds and their hybrids. Maize Genetics Cooperation Newsletter, Issue 83. |
| [9] | Liu, J., Li, J., Chen, F., Zhang, F., Ren, T., Zhuang, Z., and Mi, G., 2008, Mapping QTLs for root traits under different nitrate levels at the seedling stage in maize (Zea mays L.). Plant Soil, 305, 253-265. |
| [10] | Engels, C., and Kirkby, K.A., 2001, Cycling of nitrogen and potassium between shoot and roots in maize as affected by shoot and root growth. Journal of Plant Nutrition and Soil Science, 164, 183-191. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



