-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2015; 5(1): 9-17
doi:10.5923/j.ijmb.20150501.02
Seven Amaranthus L. (Amaranthaceae) Taxa Flavonoid Compounds from Tehran Province, Iran
Mitra Noori 1, Mehdi Talebi 1, Zeinab Nasiri 2
1Department of Biology, Faculty of Science, Arak University, Arak, Iran
2MSc student of Department of Biology, Faculty of Science, Arak University, Arak, Iran
Correspondence to: Mitra Noori , Department of Biology, Faculty of Science, Arak University, Arak, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this study root, leaf, inflorescence and seed flavonoids of 7 Amaranthus L. taxa are compared. Aqueous-ethanolic extracts of collected plant material were examined to practice flavonoid detection, isolation and identification by 2-dimensional paper chromatography, thin layer chromatography, UV spectroscopy and available references. Results showed all of examined taxa have flavonoid sulphate, flavon C & C-/O glycosides and aglycons in their root and aerial parts with the exception of leaves that had not aglycons. Isorhamnetin, Kaempferol, Quercetin and Rutin were found in all of studied taxa aerial parts. All of taxa roots had kaempferol, quercetin and rutin.
Keywords: Amaranthus L., Amaranthaceae, Flavonoid compounds, Chromatography
Cite this paper: Mitra Noori , Mehdi Talebi , Zeinab Nasiri , Seven Amaranthus L. (Amaranthaceae) Taxa Flavonoid Compounds from Tehran Province, Iran, International Journal of Modern Botany, Vol. 5 No. 1, 2015, pp. 9-17. doi: 10.5923/j.ijmb.20150501.02.
Article Outline
1. Introduction
- The genus Amaranthus L. from Amaranthaceae family consists of 60-75 species in the world [1, 2]. They are monoecious and dioecious usually annual species of worldwide distribution, about 40 of which are native to America, while the remaining ones are native to the other continents [1]. Mobayen (1979) has reported 6 species, Ghahreman (1980) more than 7 species and Rechinger (1998) 11 species in Iran that these differences are by the reason hybridization and domestication of some of them. Amaranthus species are annual herbaceous plants that grow in all of continents and throughout Iran [3-5]. A. retroflexus, A. deflexus, A. lividus var. ascendens, A. graecizans var. graecizans and A. graecizans var. sylvestris taxa have been reported for Tehran Province [5]. In today’s scenario people rely on herbal medicines for health care, because the other treatment options available are more expensive and are often associated with serious side effects. A significant number of modern pharmaceuticals drugs are based on or derived from medicinal plants. Herbal medicines are usually in the aim of vegetable drugs or there extract which primarily serves for the treatment of diseases and to maintain health or that are utilized primarily by man for the treatment of diseases or to maintain a state of improved health. Many researchers reported ethno-pharmacological and nutritional importances of Amaranthus species in their works.Nana et al (2012) studied on Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and A. hybridus L. extracts. They describe a preliminary assessment of the nutraceutical value of A. cruentus and A. hybridus, two food plant species found in Burkina Faso. The UV–VIS spectra of the hydroacetonic, methanolic and aqueous extracts of both Amaranthus species showed peaks suggesting the presence of flavonoids and betalains. Their results indicated that the phytochemical contents of the two species justify their traditional uses as nutraceutical food plants [6]. Ayethan et al (1996) reported some pharmacological properties of A. spinosus. Extracts of the leaf had been used in the treatment of menstrual disorders in man. The plant is used as a sudorific and febrifuge and is recommended for eruptive fevers. The leaves are considered a good emollient, lactogogue and a specific treatment for colic [7]. Externally, the bruised leaves are applied to treat eczema in Kenya [8]. A. caudatus is domesticated mostly for its grain [9]. In Kenya, Amaranthus hybridus leaves are eaten as spinach or green vegetables. In Nigeria, Amaranthus leaves combined with condiments are used to prepare soup [10]. These leaves boiled and mixed with a groundnut sauce are eaten as salad in Mozambique [11] or pureed into a sauce and served over (farinaceous) vegetables in West Africa [12]. The plant is used in the treatment of intestinal bleeding, diarrhoea and excessive menstruation [13]. Maiyo et al (2010) studied on phytochemical constituents and antimicrobial activity of 3 Amaranthus species (A. hybridus, A. spinosus and A. caudatus) leaf extracts on Staphylococcus aureus, Bacillus spp, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosae, Proteus mirabillis and Klebsiella pneumoniae and a pathogenic fungus Candida albicans. Results showed that these plants are traditional medicine for the treatment of infections and consumption and have ethno-pharmacological and nutritional importance [14]. Also Islam et al (2007-2010) worked on antimicrobial and irritation activities of A. viridis extracts using different extraction methods. Their result showed antibacterial range of ethanol was more prominent than that of its aqueous extract and polar mass. However, hexane extracts of the plant displayed a greater antibacterial activity against Gram positive and Gram negative microorganisms than that of chloroform extracts [15-17]. Srivastava et al (2011) studied effect of methanolic extract of Amaranthus spinosus on hematocellular components after single and daily administration by determining hematocellular indices. Their results revealed that the RBC and WBC count as well as Hb% was significantly altered due to administration of methanolic extract of Amaranthus spinosus and plant act as a good antioxidant and safe to use against various types of Blood related disorders [18]. Ashok Kumar et al (2011) studied on heaptoprotective activity of methanolic extract of Amaranthus viridis L. in paracetamol induced hepatotoxicity. Result showed that methanolic extract of whole plant of A. viridis, possess liver protective activity against paracetamol induced hepatotoxicity in rats [19]. Harsha Vardhana (2011) used A. spinosus root extracts for in vitro antimicrobial activity examination. Both ethanolic and aqueous extracts showed antimicrobial activities but ethanolic extract had the best result in comparison with aqueous extract [20]. The species are used in tropical and subtropical countries for human nutrition both as vegetables and grains and also as animal feed [21, 22]. It has been reported to have some pharmacological properties [7]. The plant have several active constituents like alkaloids, flavonoids, glycosides, phenolic acids, steroids, amino acids, terpenoids, lipids, saponins, betalains, B-sitosterol, stigmasterol, linoleic acid, rutin, catechuic tannins and carotenoids. It also contains amaranthoside, a lignin glycoside, amaricin, a coumaroyl adenosine along with stigmasterol glycoside, betaine such as glycinebetaine and trigonelline [23, 24]. Paśko et al (2008) analysed selected phenolic acids and flavonoids in A. Cruentus seeds and sprouts by HPLC. The main flavonoid found in the sprouts was rutin. Vitexin, isovitexin, and morin were also detected in the sprouts and orientin, vitexin, isovitexin, morin, and traces of hesperidin and neohesperidin in the seeds. A. Cruentus is a pseudocereals with particularly highly regarded nutritional value by the reason existing high biological significance of the flavonoids and phenolic acids in this plants [25]. Flavonoids as secondary metabolites are valuable and widely and effectively used in chemosystematics [26]. They occur widely in plant organs and are a biologically major and chemically diverse group of secondary metabolites that are popular compounds for chemotaxonomic surveys of plant genera and families [27]. Plant phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level [27, 28, 29]. Noori (2014) compared 10 population root and leaf flavonoids profiles of 5 Scirpus species from Markazi Province, Iran for introducing chemotypes. Her results showed all of studied Scirpus populations contain vitexin, luteolin, rutin and rhamnetin in their aerial parts and roots. Also morin and tricin are two separator phytochemical characters for studied samples [30]. In this study, root, leaf and seed flavonoids of seven Amaranthus taxa aqueous ethanolic extracts are reported.
2. Materials and Methods
2.1. Collection of Plant Material and Preparation
- Mature fresh leaf, inflorescence, seed and root of 7 Amaranthus species were collected from Tehran Province, Iran area during 2013 as described in Table 1. Plant identified using available references [3-5]. Samples were air dried for detection and identification of their flavonoids.
2.2. Extraction of the Plant Material
- For a comparative analysis of the flavonoids, small extracts of all the accessions were prepared by boiling 200 mg of powdered air dried leaf, inflorescence, seed and root for 2 min in 5 ml of 70% EtOH. The mixture was cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40℃, and taken up in 2 ml of 80% MeOH for analysis by 2-Dimensional Paper Chromatography (2-D PC).
2.3. Flavonoid Analysis by 2-Dimensional Paper Chromatography (2-DPC)
- For the detection of flavonoids, ca 20 µl of each of the small extracts was applied to the corner of a quarter sheet of Whatman No 1 chromatography paper as a concentrated spot (10 applications of 2 µl). The chromatogram for each sample was developed in BAW (n-BuOH-HOAc-H2O=4:1:5; V/V; upper layer), 1st direction, and HOAc (=15% aqueous acetic acid), 2nd direction, with Rutin (= quercetin 3-O-rutinoside) as a standard. After development, the chromatograms were viewed in long wave UV light (366 nm) and any dark absorbing and fluorescent spots were marked. Rf -values in BAW and 15% HOAc were calculated.
2.4. Methods of Identification of the Flavonoids
- After obtaining sufficient amounts of purified flavonoids, as in the case of the flavonoids from leaf, inflorescence, seed and root of the species, they were identified by means of UV spectroscopy using shift reagents to investigate the substitution patterns of the flavonoids [31, 32] and by acid hydrolysis to identify the aglycone and sugar moieties. Co-chromatography with standards was also performed where possible. Flavonoid standards available for comparison during the study were Apigenin, Chrysin, Genistein, Isorhamnetin, Kaempferol, Luteolin, Morine, Myricetin, Naringenin, Quercetin, Rhamnetin, Rutin, Tricine and Vitexin (all obtained commercially, Rutin from Merck, Apigenin and Luteolin from Sigma and the rest from Fluka).
2.5. Acid Hydrolysis and Identification of Flavonoid Aglycones
- A small amount of each purified flavonoid (ca 0.5 mg) was dissolved in 0.5 ml of 80% MeOH in a test tube. To this sample 2 ml of 2M HCl was added and the mixture was heated in a water bath at 100℃ for 0.5 h. The solution was cooled. 2 ml of EtOAc were added and thoroughly mixed with the aqueous layer using a whirley mixer. The upper EtOAc layer was removed with a pipette, evaporated to dryness, dissolved in 0.5 ml of MeOH and applied as spots on thin layer chromatograms (cellulose). The TLC plates were run in three solvents alongside standards to identify the aglycone moiety [33].
2.6. Data Analysis, Principal Component Analysis (PCA) and Hierarchical Cluster Analysis
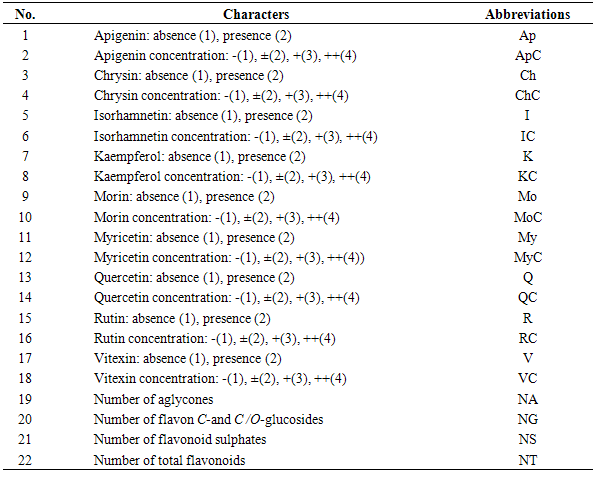
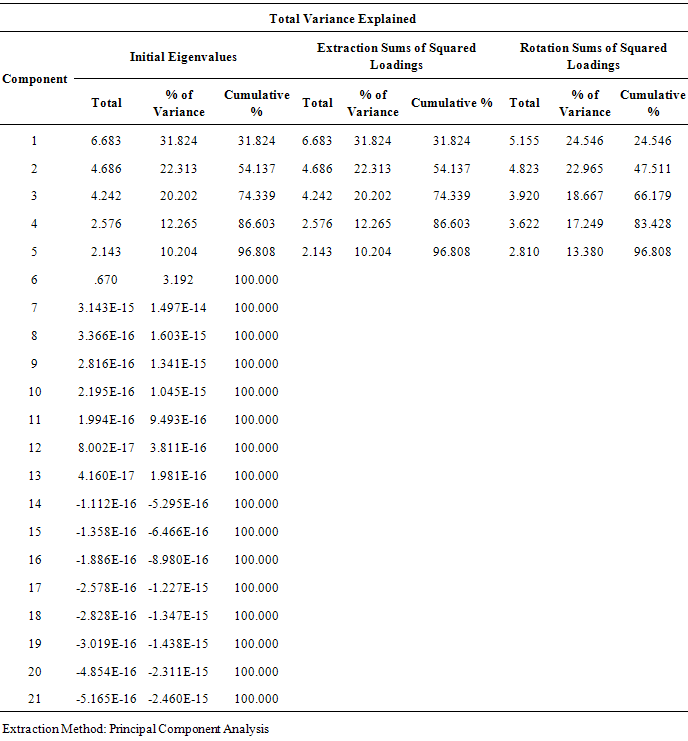
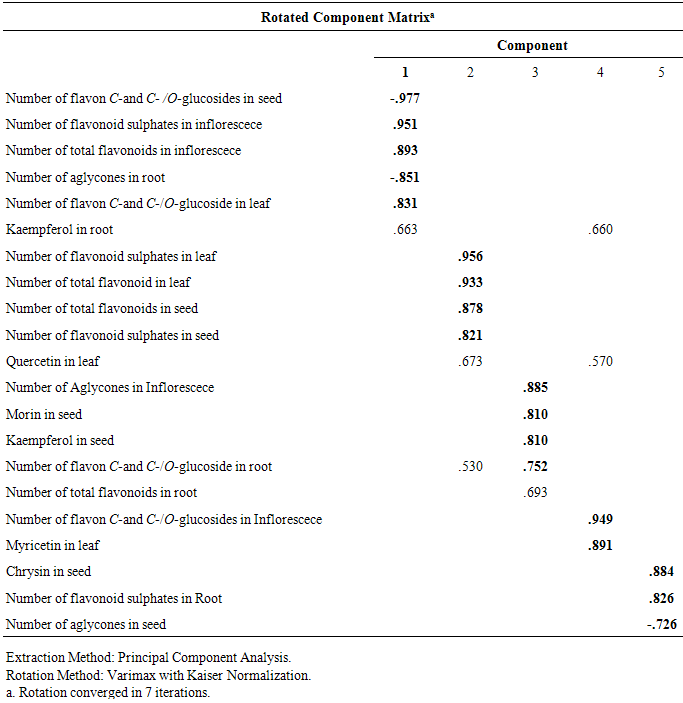
- Twenty two qualitative and quantitative phytochemical characters were studied (Table 2). Qualitative characters were coded as multistate characters and the quantitative characters were used. Data were analyzed using the SPSS for windows release 16.0 statistical package for social scientists by principal component analysis (PCA) test (Tables 4 and 5). Then cluster analysis using Ward, Average Linkage (between groups) and Median methods were performed on standardised phytochemical data. Ward method was the best. Fit of the clusters to the original data was checked using cophenetic correlation. Scored characters for cluster analysis were based on existence, variation and concentration of flavonoids (Table 1 & 3 and Figure 1).
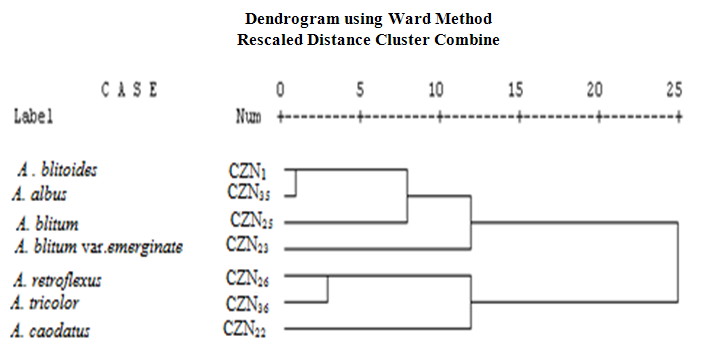 | Figure 1. Cluster analysis (Ward) of 22 phytochemical characters of studied Amaranthus in Tehran Province, Iran. Scored characters for cluster analysis have been shown in Tables 1-3 |
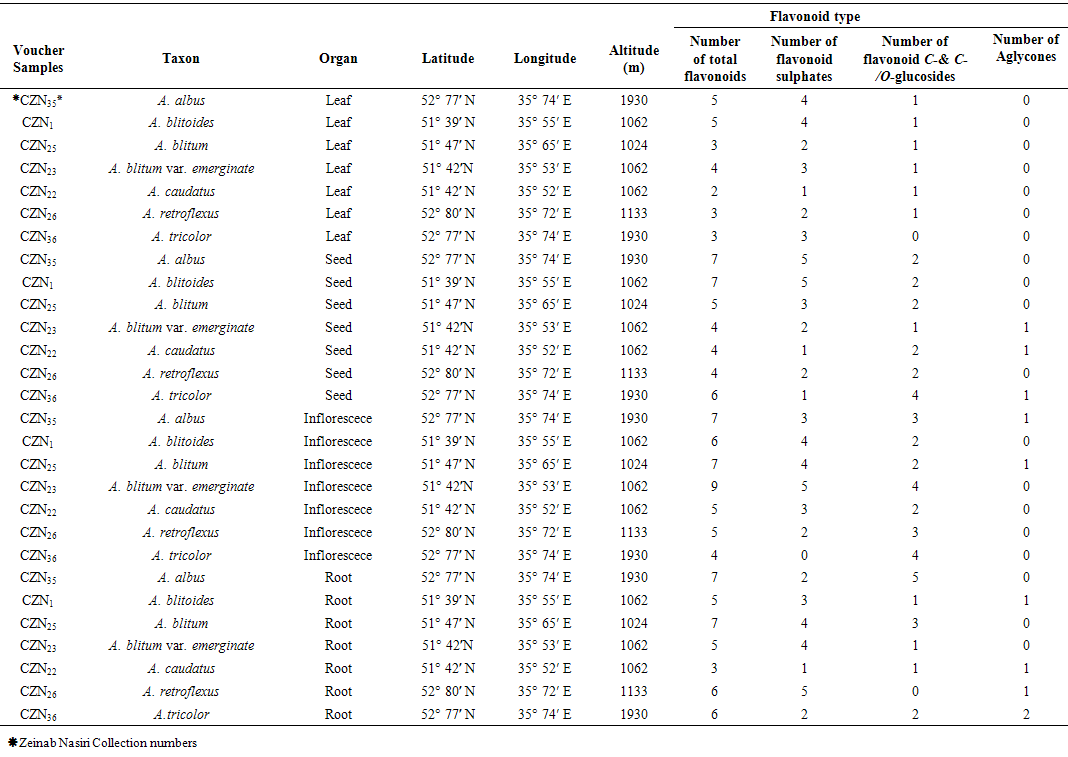 | Table 1. Collection information of 10 collected Populations of Sphecidae family from different parts of Golpayegan-Isfahan Province, Iran |
|
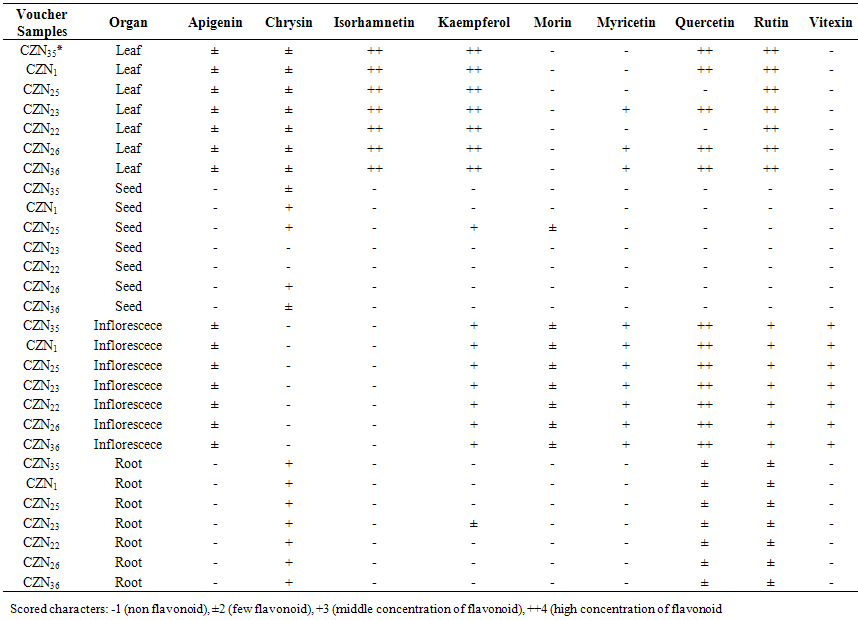 | Table 3. Thin Layer Chromatography data of leaf, inflorescece, seed and root of 7 studied Amaranthus species from Tehran Province, Iran |
3. Results
- Results showed leaf, inflorescence, seed and root of Amaranthus species contained flavonoid compounds. Data in Tables 1 and 3 show the collection information and 2-dimentional paper and thin layer chromatographical data. Their flavonoid profiles show a wide variety between the organs species. Results showed all of examined taxa had flavonoid sulphate, flavon C & C-/O glycosides in all of their examined organs with the exception of A. tricolor species that had not these two compounds in its leaf and inflorescence. Also A. retroflexus root lacked flavon C & C-/O glycosides. Aglycons were not found in all of studied species leaves. A. blitum var. emerginate, A. caudatus and A. tricolor species had aglycones in their roots while others lacked. Aglycones were found in A. albus and A. blitoides species inflorescences where as the rest species had not. A. albus, A. blitum and A. blitum var. emerginate taxa roots had not aglycones while others had. The most flavonoids number was observed in A. albus species organs and A. caudatus showed the lowest (Table 1). Kaempferol, rutin, myercetin, quercetin and vitexin were found in all of studied taxa inflorescences and probably apigenin and morin were also existed. All of the studied taxa leaves had isorhamnetin, kaempferol and rutin. Quercetin was found in all of the studied species with the exception of A. blitum and A. caudatus species. Seeds of all studied taxa with the exception of three species (A. blitum, A. blitoides and A. retroflexus) contain chrycin. Chrycin and may be rutin and quercetin were found in all of the studied taxa roots. These results show that firstly inflorescence and then leaf had the most number and variety of flavonoids in the studied Amaranthus taxa.
4. Discussion and Conclusions
- As Tables 1 and 3 show all of studied Amaranthus species contained flavonoid compounds in their roots, leaf, inflorescences and seeds. Inflorescence and then leaf had the most number and variety of flavonoids in the studied Amaranthus taxa. Many researchers reported ethno-pharmacological and nutritional importances of Amaranthus species in their works [6-22]. Our results showed all of examined taxa had flavonoid sulphate, flavon C & C-/O glycosides in all of their examined organs with the exception of A. tricolor species that had not these two compounds in its leaf and inflorescence. Also A. retroflexus root lacked flavon C & C-/O glycosides. Aglycons were not found in all of studied species leaves. A. blitum var. emerginate, A. caudatus and A. tricolor species had aglycones in their roots while others lacked. Kaempferol, rutin, myercetin, quercetin and vitexin were found in all of studied taxa inflorescences and probably apigenin and morin were also existed. All of the studied taxa leaves had isorhamnetin, kaempferol and rutin. Quercetin was found in all of the studied species with the exception of A. blitum and A. caudatus species. Seeds of all studied taxa with the exception of three species (A. blitum, A. blitoides and A. retroflexus) contain chrycin. and may be rutin and quercetin were found in all of the studied taxa roots (Tables 1 and 3). Paśko et al (2008) analysed selected phenolic acids and flavonoids in A. Cruentus seeds and sprouts by HPLC. Rutin was the main flvonoid in sprouts. Also vitexin, isovitexin, and morin were detected in the sprouts and orientin. Orientin, vitexin, isovitexin, morin, and traces of hesperidin and neohesperidin were found in the seeds. A. Cruentus is a pseudocereals with particularly highly regarded nutritional value by the reason existing high biological significance of the flavonoids and phenolic acids in this plants [25].Factor analysis results of phytochemical characters in Tables 4 and 5 showed that the first five factors describe about 97% of total variance. First component with 32% total variation was found positively correlated with total flavonoids number, flavonoid sulphates number in inflorescence and flavon C-and C-/O-glucoside number in leaf, and negatively correlated with number of flavon C-and C-/O-glucosides in seed and number of aglycones in Root. Secondary component with 22% total variation was positive and significantly correlated with leaf total flavonoids and flavonoid sulphate numbers and also with seed total flavonoids and flavonoid sulphate numbers. Third component with 20% total variation was correlated positively and significantly with inflorescence aglycones number, seed morin and kaempferol existence and number of flavon C-and C-/O-glucoside in root. Component four with 12% total variation was correlated positively and significantly with leaf myricetin existence and inflorescence flavon C-and C-/O-glucosides number. Last component with 10% total variation was positively correlated with number of flavonoid sulphate in root, aglycones number in seed and chrysin existence in seed. Figure 1 cluster analysis of phytochemistry data using cophenetic correlation showed two main clades. First main clade consists of two subclades that first one contained three species (A. blitoides, A. albus and A. blitum) and second sub-clade has just one taxa (A. blitum var. emerginatus). Secondary main clade consists of two subclades that A. retroflexus and A. tricolor species are in one group and A. caodatus has been separated from them in second group. These results and reviewing the studied plant color show that all of plant species in first main clade are completely green while examined plant species in the second main subclade have different colors for example green-red, green-pink, green-purple, pink, red or purple. It is believed that plant color is directly or indirectly correlated with secondary metabolites specially flavonoids and anthocyanins as our study results in Tables 1 and 3 showed existing flavonoid compounds in all of studied Amaranthus species in their organs (root, leaf, inflorescence and seed). Based on these results it is concluded that the quantities and presence of important metabolites such as flavonoids depend on the various parts of the plant used. Therefore, depth study of Amaranthus L. medicinal ingredients and flavonoids can provided the basis for further development and utilization. Finally, studying Amaranthus L. taxa flavonoid compounds can reveal their cophenetic correlations based on their phytochemistry properties as Noori (2014) found morin and tricin are two separator phytochemical characters for introducing chemotypes in some Iranian Scirpus populations [30]. It is believed that plant phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level [27-29].
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML