Abiodun Sunday Oyelakin, Muyiwa Segun Ayodele, Uche Vivian Onianwah
Department of Pure and Applied Botany, Federal University of Agriculture, Abeokuta, Nigeria
Correspondence to: Abiodun Sunday Oyelakin, Department of Pure and Applied Botany, Federal University of Agriculture, Abeokuta, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Pattern of secondary re-infestation and colonization of disturbed sites (Lawns) by invading plant species in the premises of Federal University of Agriculture, Abeokuta (FUNAAB) was investigated. Four study plots were chosen. The study involved a quantitative assessment of the plant population densities (rd) and frequencies of occurrence (rf) of plant species other than those earlier used to vegetate the study area. Plant population densities and frequencies of occurrence varied from plot to plot. Statistical analyses revealed that variations were statistically significant and were as a result of ecological factors and not chance occurrences. Secondary infestations were primarily by annual and perennial herbs. Euphorbia hirta (rd:13.3), Gomphrena celosiodes (rd:5.0), Vernonia cinerea (rd:4.2) and Tridax procumbens (rd:10.5)displayed potentials as first colonists. This study suggests that re-infestation and colonization potentials of the plant species determine their ability to establish themselves on an available and favorable space on a disturbed site.
Keywords:
Weeds, Secondary re-infestation, Colonization potentials, Open community, Ecology, Population changes, Plant succession, Seed dispersal
Cite this paper: Abiodun Sunday Oyelakin, Muyiwa Segun Ayodele, Uche Vivian Onianwah, Secondary Re-Infestation and Colonization Potential of Some Nigerian Weeds, International Journal of Modern Botany, Vol. 5 No. 1, 2015, pp. 1-8. doi: 10.5923/j.ijmb.20150501.01.
1. Introduction
Colonization is the process of plant establishment in an open (unvegetated) site (Burrows, 1990) [2]. Colonization depends on both the dispersal ability of a species and the local ecological conditions, which act as filters on plant establishment, selecting for those species with traits suitable for the site’s local conditions (Guisan and Rahbeck, 2011) [5]. Colonization is also the first process that occurs in introducing the initial vegetation which set up the potential for subsequent processes that occur in vegetation change. Vegetation consisits of assemblages of plant species forming a green mantle, an almost continuous and conspicuous plant cover on the land surface (Burrows, 1990) [2]. The initial event(s) which trigger(s) off a vegetation change is also responsible for the subsequent plant population’s response. Dowdeswell (1984) [3] reported that vegetation changes occur as plant populations respond to change in habitat conditions. Burrows (1990) [2] identified three major types of vegetation changes namely fluctuations, cycles and successions. Succession is a directional non-seasonal cumulative change in the types of plant species that occupy a given area through time. It involves the processes of colonization, establishment, and extinction which act on the participating plant species (Pidwirny, 2006) [10]. Most successions contain a number of stages that can be recognized by the collection of species that dominate at that point in the succession. Succession begins when an area is made partially or completely devoid of vegetation because of a disturbance (Pidwirny, 2006) [10]. Pidwirny (2006) [10] reported that the first stage of succession was characterized by the pioneering colonization of annual plant species on bare ground and nutrient poor soils. The annuals were then quickly replaced in dominance in the next year by biennial plants and grasses. After about 3 to 4 years, the biennial and grass species gave way to perennial herbs and shrubs. These plants live for many years and have the ability to reproduce several times over their lifespans. Two types of succession are recognised as primary and secondary succession (Finegan 1984 [4]; Pidwirny, 2006 [10]). Pidwirny (2006) [10] defined primary succession as the establishment of plants on land that has not been previously vegetated. It begins with colonization and establishment of pioneer species. While secondary succession is the invasion of a habitat by plants on land that was previously vegetated. Removal of past vegetation may be caused by natural or human disturbances such as fire, logging, cultivation, or hurricanes. Furthermore, he recognises variations in secondary succession and classified it into four, namely allogenic, autogenic, progressive and retrogressive successions. Finegar (1984) [4] described allogenic as a condition that occur where some stress factors cause death of prevailing population of plants and are replaced permanently by other species. While autogenic occurs when vagetation change is caused by alteration of habitat condition by vegetation. The prevailing species die and are replaced by other more competitive species. However, progressive succession is a succession where the community becomes complex and contains more species and biomass over time while retrogressive succession is a succession where the community becomes simplistic and contains fewer species and less biomass over time. Some retrogressive successions are allogenic in nature. For example, the introduction of grazing animals result in degenerated rangeland (Finegar, 1984 [4]; Burrows, 1990 [2]; Pidwirny, 2006 [10]).A means of studying colonization, as a process in vegetation change is to examine over a period, newly formed land surfaces or places where vegetation disturbance has occurred. Suitable sites of vegetation disturbance are areas where soil has been cleared, turned over or vegetation burnt. It also includes heavily grazed or damaged areas by logging (Burrows, 1990) [2]. Within a year after disturbance much of the space in the abandoned field is covered by population of mainly annual and biennial herbaceous species (Guisan and Rahbeck, 2011) [5]. Sweiringa and Wilson (1972) [12] reported that species composition are usually similar qualitatively, the flora may differ quantitatively, even between adjacent fields. In other words, in an area undergoing colonization, plant populations vary quantitatively from area to area. Many workers have reported factors responsible for plant species colonization potentials (Sweiringa and Wilson, 1972 [12]; Burrows, 1990 [2]; Kotanen, 1996 [9]; Wichmann, 2009 [13]). One of these factors that determine colonization is the role of propagules while history of site also determines what vegetative propagules of perennial plants are present after a disturbance (Burrows, 1990) [2]. The depth of a disturbance also has significance on the number of vegetative propagules left in the soil (Kotanen, 1996) [9].Seed dispersal is the movement or transport of seeds away from the parent plant (Wichmann, 2009) [13]. Plants have limited mobility and consequently rely upon a variety of dispersal vectors to transport their propagules, including both abiotic and biotic vectors. In other words, the species with higher populations and distributions were those with more effective dispersal. Colonization potentials of species are also determined by some biotic and abiotic factors. One of the biotic factors reported to be responsible for species inter-relationship within a habitat is competition (Burrows, 1990) [2]. Abiotic factors reported to be influencing species colonization are temperature intensity, duration and extremes. Soil too, is affected by plants that grow on it and in turn affects the nature of the vegetation (Burrows, 1990) [2]. Other abiotic factors include adaptability, physical and chemical variables or environmental phenomena which affect the distribution of plants. These all suggest the need to understand the infestation and colonization capabilities of plants often associated with weedy traits among Nigerian flora. The objective of this study is to investigate the population densities and frequencies of occurrence of plant species other than those earlier used to vegetate the areas under study, after the initial opening up of the site for construction of buildings. The areas fall within lawns in the University premises. This is with a view to quantifying the potentials of identified individual plant colonizer and postulating possible factors enhancing these potentials.
2. Materials and Methods
2.1. Experimental Plot Allocation Description
Four sample plots were randomly selected among the lawn areas of the Federal University of Agriculture, Abeokuta, Alabata, Ogun State, Nigeria. Plots selection method described by Kershaw and Looney (1985) [8] was adopted. This was a partial random sampling in which an area with homogenous plant populations was subdivided into convenient number of equal-sized plots. The plots used were randomly sampled from within the subdivisions. The chosen plots were located as follows:Plot 1: Lawn located in front of the University library, between the hedges of Ixora spp. leading to the College of Natural Sciences (COLNAS) building.Plot 2: Lawn between COLNAS building and the University Bus terminus.Plot 3: Lawn East of the Senate building facing the direction of the COLNAS building.Plot 4: Lawn in front of Senate building, adjacent to the University Bus terminus.Each plot was mapped out and the dimensions taken with a calibrated tape rule at 10×10m. The boundary of each plot was pegged down.
2.2. Identification and Population Evaluation of Invading Plant Species Per Plot
Plant species sampling plots were obtained through thrown (1m × 1m) quadrats in each location. Any plant other than that initially planted in a location was regarded as an invading species for identification and population evaluation. Samples of such plants were collected in polythene bags and processed for identification. Plant identification was first conducted by means of Flora and weeds literature (Hutchinson & Dalziel, 1968 [6]; Akobundu and Agyakwa, 1998 [1]) and later confirmed using Herbarium specimens at the Forestry Research Institute of Nigeria (FRIN) and Obafemi Awolowo University (OAU) Ile-Ife, Nigeria.
2.3. Estimation of Plant Population Densities (PPD) of Identified Plant Species
Data on PPD were collected by random sampling. This was done by using a 1×1 meter quadrant frame. Walking in a straight line from one point of a plot to the other end and throwing the quadrant every five paces. Records of the number of individual plants per species were taken wherever the quadrant landed (Slingsby and Cook, 1986 [11]). The PPD was estimated as the count of individual plant per unit area. Records were taken of ten quadrants per plot.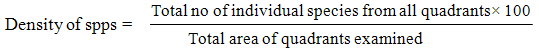
2.4. Evaluation of Frequency of Occurrence of Identified Plant Species
Data on frequencies were collected by recording whether any species being considered was present or not. The frequency was the measure of the chance of finding a species with any throw of a quadrant, (after Slingsby and Cook, 1986 [11]).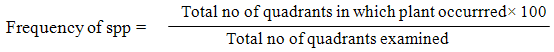
2.5. Analysis of Data
The means of plant population densities (PPD) and the frequency values of each species were evaluated. The variations in plant species that occurred in the different plots would be shown to be as a result of ecological factors rather than due to chance, through a test of statistical significance.The statistical test for significance used was the one way classification of variance. This was done to test if there was a statistical (ecological) significant difference at P<0.05. The conclusion can be drawn if the variations were caused by ecological factor(s). At P>0.05, if there was no statistical (ecological) significant difference. The conclusion can also be drawn that the variations were due to chance.P = Probability at 5% or 0.05 level of significance.
3. Results
Prior to the establishment of the permanent site of the Federal University of Agriculture, Abeokuta (FUNAAB), the area was predominantly a Secondary Rain Forest with some areas in transition between derived savannah grassland and a secondary forest. The site was cleared in 1994 and areas designated to be lawns were sand-filled with top soil and grassed with species of aesthetic purpose. Species of grass were propagated selectively per site based on the species suitability for the particular topography. Hardier species were planted on sites liable to erosion by wind or run off water. Cynodon compressus (Carpet grass) was planted on plots 1 and 2 forming a flat plane landscape open community while Cynodon dactylon (Bahama or Portharcourt grass) was on plots 3 and 4 which had some prominent undulations sufficiently susceptible to runoff erosion.
3.1. Identified Invading Plant Species on Experimental Plots
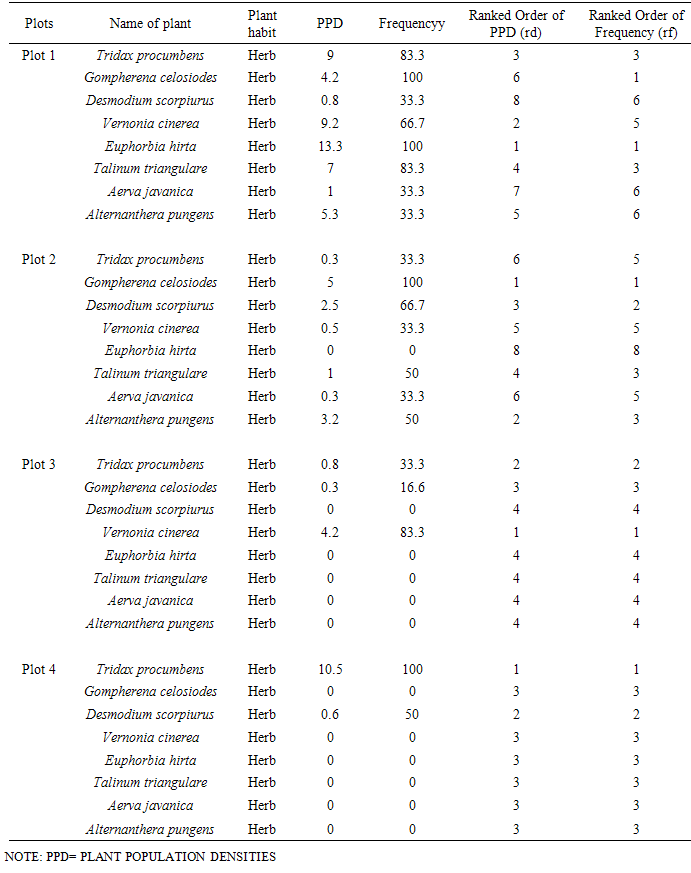
It was observed that most of the grass species planted were not well established because they were out-competed with hardier, more vigorous wild plants that dispersed there. A not too efficient maintenance of the cultivated grasses must have encouraged invasion by wild species as evidenced by the unkept status of the plots before each periodic mowing operations which was on an average of once bi-monthly. On all lawns, the original species planted can be observed predominantly along the edges of the lawns. Numerous other plants of different growth habits, annuals and perennials were observed on the plots. The top eight prominent species among them (Table 1) were Tridax procumbens, Gomphrena celosiodes, Desmodium scorpiurus, Vernonia cinerea, Euphorbia hirta, Talinum triangulare, Aerva javonica and Alternanthera pungens. Species with scanty representation on the plots were discarded for analysis and reporting. Table 1. Plant population densities and frequencies (Plot 1-4)
 |
| |
|
3.2. Mean of Plant Population Densities (PPD) of Identified Species
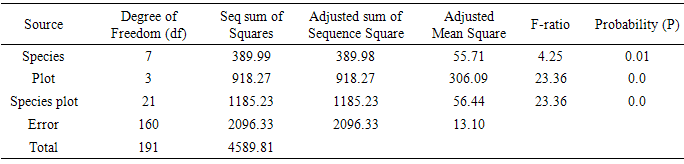
It was observed that different invading species prevalently dominated Plots 1, 2, 3 and 4 in notable densities. Of the eight prominent species recorded in this study, only four wrestled for the top position of dominance on the different plots namely: Euphorbia hirta (PPD 13.3) on Plot 1; Gomphrena celosoides (PPD 5.0) on Plot 2; Vernonia cinerea (PPD 4.2) on Plot 3 and Tridax procumbens (PPD 10.5). The value of dominance of these species in terms of population densities on the various plots is noteworthy. There were more of the species co-invading plots 1 and 2 than there were in plots 3 and 4 (Table 1). In actual fact, some of the species were absent in some plots, with only two or three species co-invading as found in plots 3 and 4 (Table 1). There was significant difference at P=0.00<0.05, for plant population densities.
3.3. Mean of Frequencies of Occurrence of Identified Species
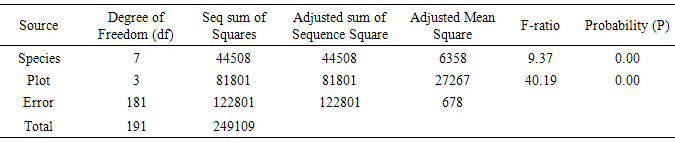
Species with highest population densities (rd) in each plot also manifested the highest value of frequency of occurrence (rf) on the plot (Table 1). However, some species with low population densities were found to occur more frequently in the plots while some species with high population densities had low frequencies of occurrence. Euphorbia hirta, Gomphrena celosiodes, Vernonia cinerea and Tridax procumbens maintained a record of high frequency of occurrence with a high population density on their colonized plots. The prevalence of Tridax procumbens on all the 4 plots and its pronounced performance on Plot 4 where most other species were absent is particularly noteworthy. There was significant difference at P=0.00<0.05, for plant frequency of occurrence.
4. Discussion
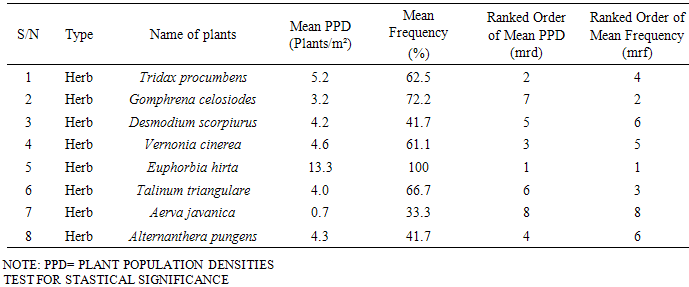
Data collected on eight prominent species among others found invading disturbed plots, qualified them for selection for scrutiny on their potentials in propagule dispersal and colonization potentials of areas infested. In the study, the top eight plant populations common on four different consecutive plots investigated consisted of 5 annual and 3 perennial herbs. This trend corroborates the assertion by Keever (1950) [7] who reported that within a year after disturbance, much of the space in an abandoned field is covered by population of mainly annual and biennial herbaceous species. Burrows (1990) [2] also reported annual herbaceous weeds as the first colonists in a site where vegetation has been disturbed. Of the eight species identified in this study, Euphorbia hirta, Gomphrena celosiodes, Vernonia cinerea and Tridax procumbens have proven themselves as swift “first colonists” described by Keever (1950) [7] and Burrows (1990) [2] on the available space of a disturbed open community as a result of regular field mowing.The trend observed for infestation and colonization of open spaces in the plots by these plants indicates quantitative differences in plant populations per species from plot to plot (Table 1). This pattern agrees with Swieringa and Wilson (1972) [12] who reported that during colonization of disturbed sites, Flora may be similar in composition but differ quantitatively even between adjacent fields. The interplay of two critical features namely: the dispersal ability of a plant and the local ecological conditions are germane in the colonization potential of a species (Guisan and Rahbeck, 2011) [5]. Burrows (1990) [2] opined that species with higher population per distribution are well armed with effective dispersal potentials. However, the local ecological conditions act as filter as to which of such species get established as effective colonizers (Guisan and Rahbeck, 2011) [2]. This must explain the reason behind a species like Euphorbia hirta which had the best combination of rd and rf on plot 1, but failing to be found on the other plots.Results from one-way classification of variance indicate that quantitative differences in population densities among the experimental plots were statistically different (Table 3). It could therefore be deduced that these differences were not chance occurrences but due to ecological factors such as dispersal, adaptability, light, water and other edaphic factors. This confirms the fact that though some plants manifest proficient infestation in the process of invasion of a disturbed site, but get filtered off without the favour of ecological determinants of colonization. This is quite evident in the observation of the performances of these top eight species in plots 1 and 2 (a flat plane landscape open community) compared to plots 3 and 4 which had some prominent undulations susceptible to runoff erosion. The combined quality value of rd and rf for the four apparently fast colonizing species namely Euphorbia hirta, Gomphrena celosiodes, Vernonia cinerea and Tridax procumbens in their various plots reported above, show that the removal of whatever ecological factors inhibiting their performance in other plots will enhance their occurrence in prolific populations there.Table 2. Mean plant population densities and frequencies from four plots
 |
| |
|
Table 3. Plant population densities
 |
| |
|
Table 4. Frequencies of all species
 |
| |
|
Table 5. Botanical details of top 8 species identified on the plots
 |
| |
|
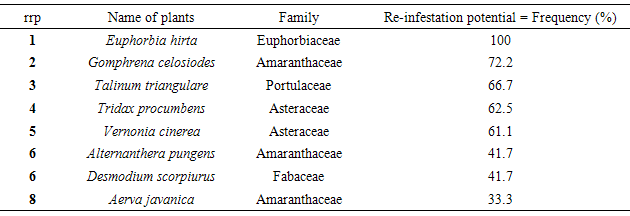
Table 6. Ranked order of in-infestation potential
 |
| |
|
5. Conclusions
The re-infestation and colonization potentials of the plant species (weeds) will be the species ability to re-infest or re-colonize as much available space on the disturbed site. If the colonization potential of a plant species is to be considered as its ability to establish itself on as much available space, results of this study indicate this, as species mean frequency reflecting its distribution round the site. Among the species considered, Euphorbia hirta (Family Euphorbiaceae) an annual (grass) had the highest potential to re-infest and colonize the study area (Table 6).
References
| [1] | O. Akobundu and C. W Agyakwa. A Handbook of West African Weeds. 2nd ed. Ibadan, Nigeria: International Institute of Tropical Agriculture (IITA) 1998. |
| [2] | C. T. Burrows. Processes in vegetation change. 3rd ed. London, UK: Union Hyman Ltd. 1990. |
| [3] | W. H. Dowdeswell. Ecology principles and Practice. 1st ed. London, UK: Heinemann Educational Books Ltd. 1984. |
| [4] | Finegar, M. 1984. Forest Succession. Nature 312, 109-110. |
| [5] | Guisan, A., and Rahbeck, C., 2011, SESAM: A new framework integrating macro ecological and species distribution models for predicting spatial-temporal patterns of species assemblages, J. Biogeography, 14, 33–44. |
| [6] | J. J. Hutchinson, and J. M. Dalziel, Flora of West Tropical Africa. Avi Publishing. Westport 2nd ed.vol.11. Reviewed by Keay, R.W.J. Crown Agents, London 1963. |
| [7] | Keever, C., 1950, Causes of succession on old fields of the piedmont, Eco Monographs, 20, 229-250. |
| [8] | A. L. Kershaw and I. H. Looney, Quantitative and dynamic plant ecology. 3rd ed. London, UK Edward Arnold Publishers Ltd, 1985. |
| [9] | Kotanen, P. M., 1996, Revegetation following soil disturbance in a Califonia Meadow. The role of propagule supply. Decologia 108, 652-662. |
| [10] | M, Pidwirny, M. (2006). "Plant Succession". Fundamentals of Physical Geography, 2nd ed. Date Viewed. http://www.physicalgeography.net/fundamentals/9i.html. |
| [11] | Singsby, D and Cook, C., 1986, Practical Ecology. 1st ed. London, UK: Macmillan Educational Ltd. |
| [12] | Swieringa, S., and Wilson, R. E., 1972, Phenodynamic analyses of two first-year old fields. Amer J. Bot 55, 367-372. |
| [13] | M. C. Wichmann, M. J. Alexander, M. B. Soons, S. Galsworthy, L. Dunne, R. Gould, C. Fairfax, M. Niggemann, R. S Hails, and J. M. Bullock, "Human mediated dispersal of seeds over long-distances". Proceedings of the Royal Society 2009 276 (1656): 523–532. doi.10.1098/rspb p.1131. |

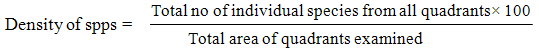
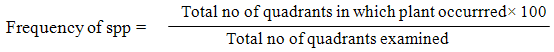
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




