-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2014; 4(2): 29-39
doi:10.5923/j.ijmb.20140402.01
Comparative Phytochemical Constituents and Pharmacognistic Importance of the Vegetative Organs of Some Phyllanthus Species in South Eastern Nigeria
Awomukwu D. A.1, Nyananyo B. L.2, Onukwube N. D.3, Uka C. J.4, Okeke C. U.4, Ikpeama A. I.5
1Department of Biology/Microbiology, Abia State Polytechnic, P.M.B. 7166, Aba, Abia State, Nigeria
2Department of Plant Science and Biotechnology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
3Department of Chemistry/Biochemistry, Abia State Polytechnic, P.M.B 7166, Aba, Abia State, Nigeria
4Department of Botany, Nnamdi Azikiwe Univerisity, Awka, Anambra State, Nigeria
5National Root Crop Research Institute, Umudike, Umuahia, Abia State, Nigeria
Correspondence to: Awomukwu D. A., Department of Biology/Microbiology, Abia State Polytechnic, P.M.B. 7166, Aba, Abia State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
An assessment and investigation into six species of Phyllanthus namely P. amarus Schum and Thonn, P. urinaria Linn., P. odontadenius Mull-Arg., P. niruroides Mull-Arg., P. mullerianus (O. Ktze) Excel and P. discoideus (Baill) Mull-Arg. belonging to the family of Euphorbiaceae were carried out with the aim of identifying and quantifying the bioactive components of these medicinal plants. The alkaloid, flavonoid, saponin, phenol and tannin contents of the vegetative parts of these plants viz. the leaves, stems and roots were screened and compared. Result from the phytochemical analyses showed that alkaloids, tannins, flavonoids and saponins were all present in the leaves, stems and roots of the studied species. Phenols were, however only absent in the stems of P. amarus, P. urinaria and P. odontadenius and roots of P. urinaria but present in the leaves, stems and roots of the rest species. All the species investigated contained appreciable amount of alkaloids, tannins and flavonoids and saponins ranging from (0.14 ± 0.06% - 3.78 ± 0.51%), (0.15 ± 0.03% - 1.82 ± 0%), (0.13 ± 0.03% - 1.59 ± 0%) and (0.14 ± 0.01% - 1.74 ± 0.01%) respectively. Phenols present ranged from (0.08 ± 0% - 0.52 ± 0%). These analytical results suggest the plants have a significant role in phyto-medicine. The importance of these plants was discussed in line with the role they play in ethno-medicinal life of the people.
Keywords: Phyllanthus species, Phytochemical constituents, Medicinal properties
Cite this paper: Awomukwu D. A., Nyananyo B. L., Onukwube N. D., Uka C. J., Okeke C. U., Ikpeama A. I., Comparative Phytochemical Constituents and Pharmacognistic Importance of the Vegetative Organs of Some Phyllanthus Species in South Eastern Nigeria, International Journal of Modern Botany, Vol. 4 No. 2, 2014, pp. 29-39. doi: 10.5923/j.ijmb.20140402.01.
1. Introduction
- In many countries around the world, Phyllanthus species are used in folk remedies [1]. The genus Phyllanthus has a long history of use in the treatment of liver, kidney and bladder problems, diabetes and intestinal parasites [2]. The name “Phyllanthus” means “leaf and flower” because the flower, as well as the fruit, seems to become one with the leaf other common names includes gripe weed, stonebreaker, leaf flower and among others [3]. The medicinal values of these plants lie in some chemical substances that produce a definite physiological action on the human body. The most important of these substances are alkaloids, glycosides, tannins, flavonoids, saponins, phenols, oils and many others [4]. These are very useful in economic botany, medicinal chemistry and pharamacognosy, some drugs have been obtained from natural sources while some may be prepared by the modification of the natural ones [5]. According to Rahila et al. [6], there is need for the local herbs to be evaluated for phytochemicals so as to determine the potential of indigenous sources of medicines.Nigeria is one country rich in raw and useful herbs from which important drugs could be prepared or agent which serve as starting products for the potential synthesis of drugs [7]. Most of these plants used for traditional medicine are equally consumed by humans in Nigeria, but their medicinal values are not determined. The biosynthesis of secondary metabolites varies among plants, even in different organs of plants and their biosynthesis depends on the environmental factors in which they grow. Intra-specific variation in phytoconstituents has been documented extensively among the plants [8] [9]. According to Thyagarajan et al. [10] plant extracts from Phyllanthus have beneficial effects on liver functions. Mehrata et al. [11] and Uander and Blumberg [12] showed, using in vitro studies that P. amarus extracts (polar fractions) also have antiviral activity and are a potential remedy for hepatitis –B viral infection. Since the extract of Phyllanthus has a long history of use in tropical countries in indigenous medicine for the treatment of liver ailment, they were examined during several researches.The importance of medicinal plants has been elucidated by Edeoga et al. [13] [14] and their importance in the pharmaceutical industry. These medicinal plants have been underutilized in orthodox medicine but have confirmed to be used worldwide in the pharmaceutical, food, cosmetics and perfume industries [15].Alkaloids are very important in medicine and constitute most of the valuable drugs. They have marked physiological effect on animals [5] and show considerable pharmaceutical activity [16]. Alkaloids are stimulants acts and by prolonging actions of several hormones which require phosphodiestrerase [17] though are poisonous to cattle [18]. Tannins are useful in medicine because of their astringent properties. Tannins and Alkaloids are known to have anti-herbivore defense functions in plants [19]. Thus, the presence of tannins and alkaloids in medicinal plants could be serving as a deterrent to grazers [5]. Herbs that contain tannins are recommended for a wide range of treatments including inflammation, liver injury, kidney problems, arteriosclerosis, hypertension, stomach problems and inhibition of active oxygen and are commonly recommended as diuretics, anti-diarrheas and haemostatic [20].Saponins are glycosides widely occurring in a variety of plants and are characterized by their bitter taste and foaming in aqueous solution. They prevent disease invasion of plants by parasitic fungi [21]. Steroidal saponins from various studies indicate their importance and the interest in pharmacy due to their relationship with such compounds such as sex hormones especially in development of the female contraceptive pills [22]. In medicine, it is used to some extent as an expectorant and emulsifying agent [23].Flavonoids are the commonest phenolic constituents having 15-compounds generally distributed throughout the plants kingdom [19]. Some flavonoids have antibacterial function with gram-positive species more sensitive to isoflavanones, than their negative counterpart. Flavours are related to flavonoids and they promote particular tastes to prepared foods. The presence of flavonoids in plants have shown some effects like antibacterial, antiviral, antitoxin, antioxidant, anti-inflammatory anti-carcinogenic activities [24]. Isoflavanones, isoflavans, isoflavonones are extremely fungal pathogens [25]. They act as allelochemicals widely used in insecticides and in treatment certain physiological disorders and disease control.Phenols are synthesized via the shikimic acids pathway. Phenolic compounds are known to have anti-fungal and anti-microbial effects. Phenolic compounds believe to be active ingredients in the herbicide found up as well as some other commercial herbicide formulation [2].Several chemical investigations have been conducted where the structures of most of the phytochemicals were determined by UV, IR, Mass and NMR spectroscopy. Following the current research in phytochemicals, the secondary metabolites present in Phyllanthus amarus are alkaloids, flavonoids, hydrolysable tannins, major lignans, polyphenols [1] [26] [27] but no work has compared the chemical constituents of other species of Phyllanthus in Eastern Nigeria. Some of these species are P. urinaria Linn., P. mullerianus (O. Ktze), P. discoideus (Baill) Mull–Arg., P. niruroides Mull–Arg., and P. odontadenius Mull-Arg. [28]. It is in attempt to fill the gap on our present knowledge that this work was done. Thus, this study investigates the fundamental scientific bases for the use of these taxa by contributing to the list of plants used for ethnobotanical and medicinal purposes and to qualify and quantify the crude chemical constituents present in the plant species. The work will also give clues in aiding other scientist who may use these plants for other purposes, such as nutritional and pharmaceutical rather biological studies. This may also review works done by other scientist.
2. Materials and Methods
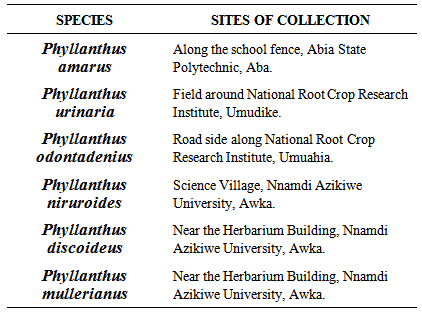
- Collection of plant materialsMature plants of the six species P. amarus, P. urinaria, P. niruroides, P. odontadenius, P. mullerianus and P. discoideus were collected from different locations of Eastern Nigeria by various investigators as in Table 1. Only healthy, fresh and succulent parts of the plants were collected. The six specimens were identified and authenticated at the Herbaria of the Department of Plant Science and Biotechnology, Michael Okpara University of Agriculture, Umudike and the Department of Botany, Nnamdi Azikiwe University, Awka. Herbarium specimens were also studied at the various institutions as well making reference to the Flora of West Tropical Africa by Hutchinson and Dalziel [29].
|
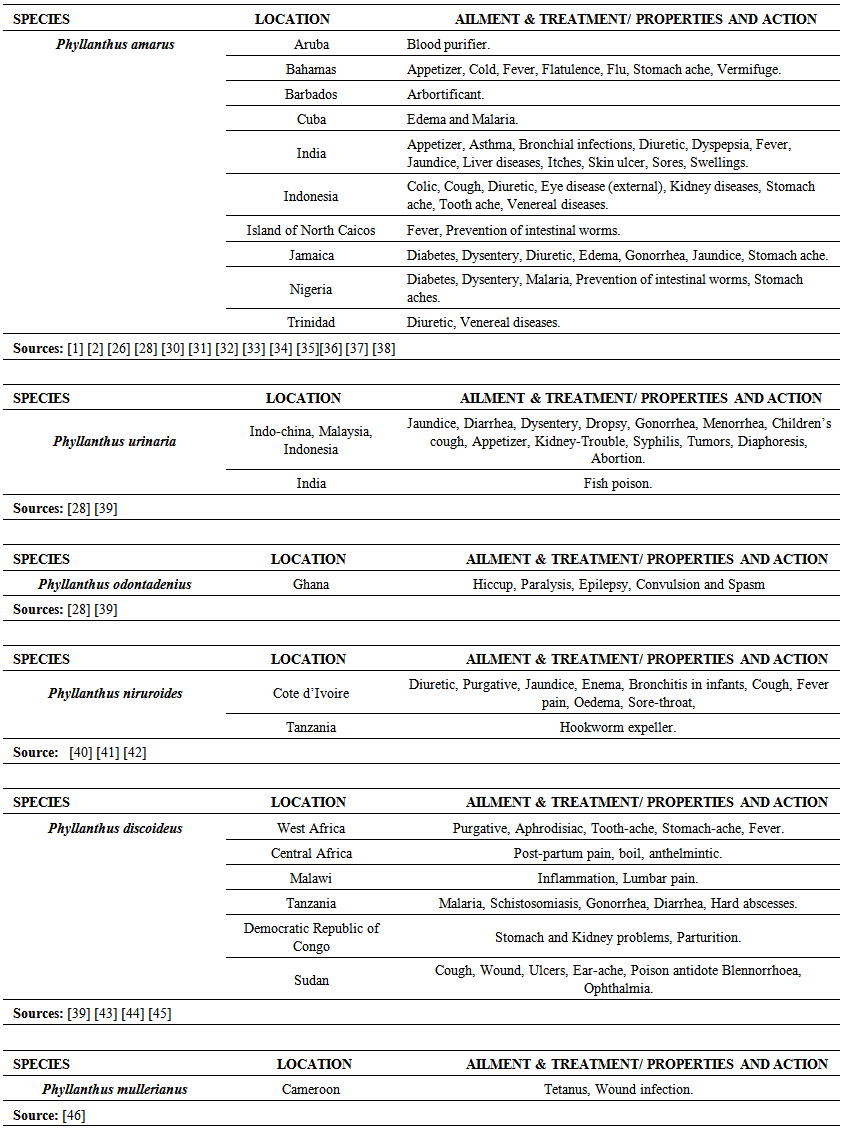 | Table 2. Ethnobotanical uses of the studied Phyllanthus species around the world |
3. Results
- The qualitative analysis and the quantitative estimation of the percentage crude chemical constituents in Phyllanthus spp studied are summarized in Table 3 and Chart 1-5. Alkaloids were very highly present in the leaves of P. niruroides. They were found to be highly present in the leaves of P. amarus and P. odontadenius, stems of P. niruroides and in the leaves, stems and roots of P. mullerianus and P. discoideus. Furthermore, they were found to be fairly present in the leaves, stems and roots of P. urinaria, stems and roots of P. amarus and P. odontadenius and in the roots of P. niruroides.
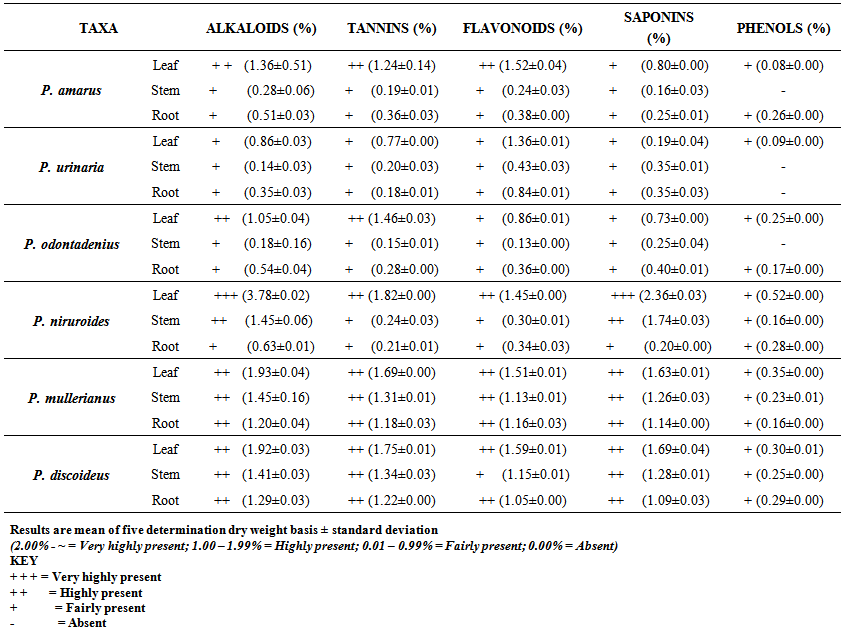 | Table 3. Qualitative analysis and percentage of the investigated crude phytochemical constituents in the studied Phyllanthus species on dry weight basis |
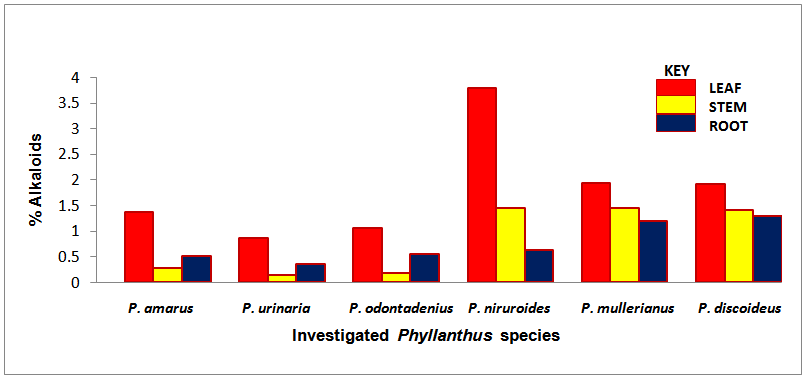 | Chart 1. Percentage alkaloids of the screened Phyllanthus species |
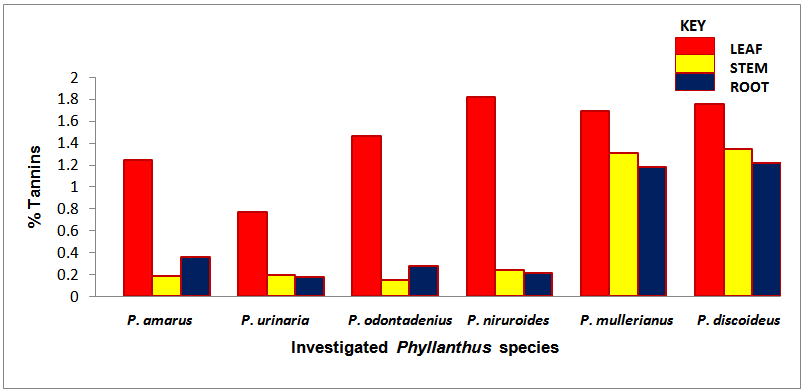 | Chart 2. Percentage tannins of the screened Phyllanthus species |
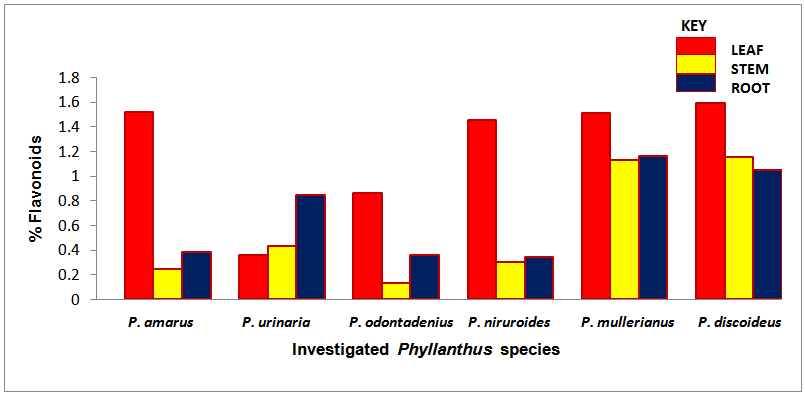 | Chart 3. Percentage flavonoids of the screened Phyllanthus species |
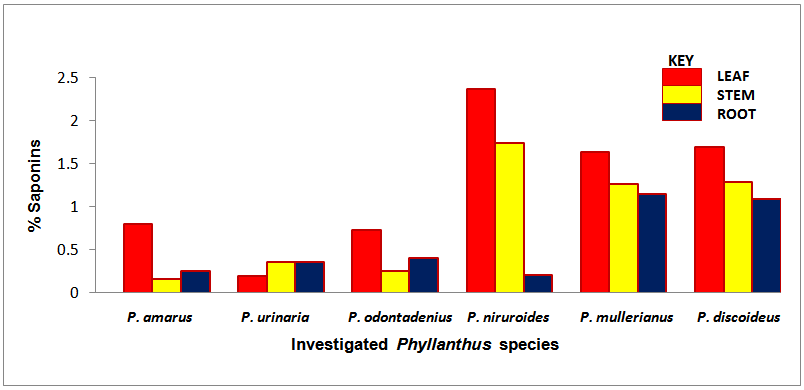 | Chart 4. Percentage saponins of the screened Phyllanthus species |
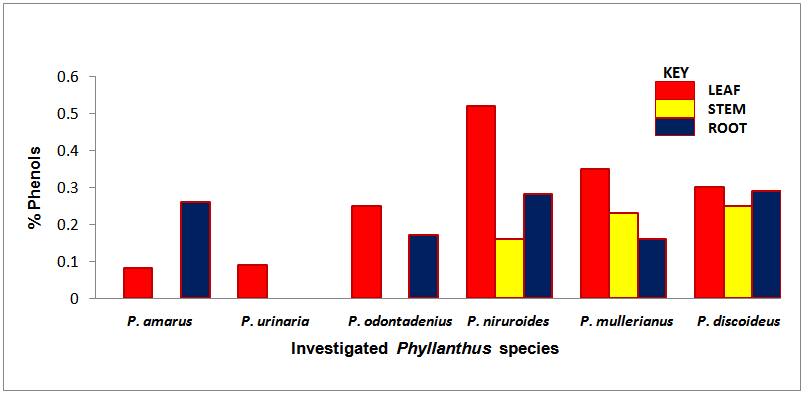 | Chart 5. Percentage phenols of the screened Phyllanthus species |
4. Discussion
- The occurrence of bioactive ingredients and the quantitative estimation of the percentage crude yield of chemical constituents of the Phyllanthus species studied therefore suggest that the leaves, stem and roots were rich in alkaloids, flavonoids, tannins and saponins except for phenols that were contained in trace amount in the leaves and roots and totally absent in some of the stems of the studied species. They were known to show medicinal activity [7].In recent times, phytochemicals are promoted for the prevention and treatment of many health conditions [52] as well as their physiological activity which facilitate natural healing with little or no side effects. The presence of these phytochemicals provide scientific explanation of the long use, recommendation and activities of Phyllanthus species as, antibacterial, anti fungal, anti diarrhoea, anti dysentery, anti protozoan and anti malarial [28].The leaves of P. niruroides had the highest percentage of alkaloids (3.78%) followed by the leaves of P. mullerianus (1.93%). The presence of alkaloids agrees with the opinion of Houghton et al. (1996) who noted there were securine, eipubbialine an isobubbaline types of alkaloids. Alkaloids are known to have a powerful effect on properties and animal physiology [5]. They are used in medicine especially the steroidal alkaloids and also show considerable pharmacological activity [16]. This explains the reason why they could not be used to feed livestock because the presence of alkaloids makes it poisonous to cattle [18]. Alkaloids are known to play some metabolic roles and control development in living system. They also have protective functions in animals and are used in medicine. Phyllanthus species are known for their potent pharmacological properties [53]. Pure isolated plant alkaloids and their synthetic derivatives are known for their analgesic, anti plasmodic and anti bacterial effects [54]. This may be the reason why the aerial parts of P. amarus are used in herbal medicine to treat malaria, stomach ache, liver ailment, kidney stone and gall bladder infection. This may also explain why the infusion of leaves from P. odontadenius is used in the treatment of paralysis, epilepsy, convulsion and spasm. Furthermore, the alkaloid contents in P. discoideus may be the reason why the plants have been recorded to have aphrodisiac effect, treatment of toothache, malaria, fever, ear-ache and lumbar pain [43] [45]. In recent years, attention has been focused on alkaloids with anti-tumorous effect [55]; this may be why leaves of P. urinaria are used in the treatment of tumors and related ailments.The leaves of P. niruroides also contained the highest percentage yield of tannins (1.82%) followed by the leaves P. discoideus (1.75%). The presence of tannins in these plants may also be the reason why most animals do not graze on the plant. This supports the opinion of Harborne [19], who pointed out that tannins have anti-herbivore function in plants and inhibit pathogenic fungi [5]. Also, tannins and alkaloids have been documented to show anti-herbivore defense function in plant [56] [57] and this could explain why the leaves of the studied Phyllanthus species are hardly grazed by herbivores. Rather, the plants show strong herbicidal potential as they repel fleas, moths and other insects [58]. In the past, it was thought to counteract poison. Thus, the presence of tannins and alkaloids in these plants could be serving as deterrence to grazer. The presence of hydrolysable tannin or pyrogallol tannins [26] in P. amarus has proved why it is recommended for a wide range of treatment including inflammation, liver injury, kidney problems, arteriosclerosis, hypertension, diuretics, diarrhoea, haemostatic, dysentery and menstrual disorder [59]. This can explain why some extracts from the roots can be used for constipation and treatment of jaundice [35], and also why the leaves of P. niruroides and P. discoideus are widely used as purgatives, treatment of jaundice, stomach ache, kidney problems, oedema, ulcer and as a poison antidote [39] [40] [41] [44]. Tannins are known to be organic substances of diverse composition with pronounced astringent properties that promote the healing of wounds and inflamed mucous membranes [55] [60]. This may be the reason why P. mullerianus is used for wound infection and tetanus (Webster, 1994). High levels of tannin in diet have reported to cause growth depression. Tannins also have the potential to complex divalent ions such as Zinc, Iron and Copper etc resulting in their unavailability [60] and have been reported to form complexes with digestive enzymes thus reducing the digestibility of proteins in foods [61].The leaves of P. discoideus recorded the highest percentage of flavonoids (1.59%) followed by the leaves of P. amarus (1.52%) although flavonoids are contained in reasonable amount in all the investigated parameters of the medicinal plants. This may be the reason why they have anti-bacterial and anti-malarial function [25] [32]. Flavonoids are known to perform very many finctions such as anti oxidant, anti allergic, anti carcinogenic, anti microbial, hepatoprotective and anti viral abilities [62]. The appreciable flavonoid contents of P. amarus is significant enough, and therefore supports the pharmacological implications shown by the plant especially in the treatment of hepatitis –B viral infection, diabetes, kidney disorders, flu, phlegm, asthma, influenza [1]. Also, the high quantity of flavonoids in P. discoideus may also prove the fact why it is used in the treatment of cough, fever, gonorrhoea, diarrhoea and ophthalmia [43]. Flavonoids are related flavours. This may also explain why the plant decoction work as an appetizer and also used in herbal teas after labour in Suriname [33] [34] [36]. Flavonoids can act as metal chelator in the cells and selectively reduce or kill cancer cells with high influx of ion [33]. Likewise, Phyllanthus teas containing flavonoids may have health promoting properties. The presence of flavonoids help to prevent platelets sickness and hence platelets aggregation [63].The leaves of P. discoideus not only contain the highest alkaloids and tannins, it also contained the highest percentage of saponins compared to other plants, having 2.36% respectively. The low percentage of crude yield of saponins in P. urinaria agreed with the opinion of Houghton et al. [32] who noted that saponins are in trace quantity in the plant species. Saponins are often referred to as “natural detergent” because of their foamy natural and have anti carcinogenic properties, immune modulation activities and regulation of cells proliferation as well as health benefit such as inhibition of the growth of cancer cells and cholesterol lowering activity [64]. The presence of saponins may be the reason why the leaves of P. urinaria are used as a fish poison in India, Indo-China, Malaysia and Indonesia [28]. Saponins serve as natural antibiotics which help the body to fight infections and microbial invasion [54]. Saponins have been recorded to prevent disease invasion of plants by parasitic fungi and has shown to affect body and liver weight, urine, plasma, faecal output and liver cholesterol concentration [41]. This can be attributed to the fact why P. amarus is used as an ingredient for diabetes, liver ailment, bladder and kidney disorder, cramps and uterus complaints (with other herbs) [33]. The high level of saponins in P. niruroides and P. discoideus may also be the reason why they are used as worm expellers and anthelminthics in the treatment of schistomiasis and related ailments [41]. Saponins are also used in the manufacture of shampoos, insecticides and various drug preparation and synthesis of steroid hormones [65]. This can be attributed to the reason why the decoctions are used in herbal baths after labour in Suriname due to the presence of saponins and flavonoids [33] [34] [36]. Saponins are known to make bronchial secretion more liquid, reduce the congestion of the bronchi and ease coughing [54]. This may also be the reason why P. amarus is used in herbal medicine for treatment of flu and asthma in combination with other herbs [34]. P. amarus plant is thus a very useful ingredient in treatment of bronchial infection [26]. The occurrences of steroidal saponins from various studies indicate the importance and the interest of saponins in pharmacy due to their relationship with such compounds that act as sex hormones especially in development of the female contraceptive pills. This may also be attributed to the fact why P. discoideus is used during parturition, treatment of blennorrhoea and post-partum pain [44]. Other characteristics of saponins include haemolytic activity, cholesterol binding properties and bitterness [32].Generally Phyllanthus species have low phenolic contents with the highest percentage yield recorded in the leaves of P. odontadenius (0.52%) followed by the leaves of P. mullerianus (0.25%) respectively. Phenols are recorded to be absent in the stems of P. amarus, P. urinaria and P. odontadenius or may be contained in trace quantity but generally low in the leaves and roots. The presence of phenolic compound in the plant proves that they have anti microbial and anti fungal effect [24]. Also plants that contain phenol could be used as anti inflammatory, immune enhancers and hormone modulators [66]. Phenols are also known to have the ability to block specific enzymes that cause inflammation and to prevent disease [60]. The presence of phenols in the leaves of P. discoideus may be attributed to the reason why extracts from it can be used for the treatment of inflammation in Malawi [45]. Phenolic compounds are well known potential phytotoxins [67] and exist as free forms, esters or as glycoside when combined with sugars. Such compounds contribute to the bitter taste, flavours and colour of foods [57].
5. Conclusions
- The present research on phytochemicals in Phyllanthus species studied has not only revealed the medicinal potential of these plants as employed by indigenous people but also their attempt to phytochemically characterise the taxa as it is commonly used in traditional medicine among Nigerians. Further studies should be carried out in order to isolate and identify, characterize and elucidate the structures of these bioactive principles and ace their potential usefulness as pharmaceutical raw material in the production of drugs. It is known and used by people for ethnomedicine though traditionally. However, the presence of various phytochemicals supports its medicinal use. The anti microbial activities of this plant against indigenous pathogenic organisms is worthy of investigation as only a few records of such exist at the moment and to assess the efficacy of these plants for the treatments of diseases as claimed by traditional healers. The diversity of chemical constituents level of toxicants and toxicity and physiology of these plants investigated also requires further investigation. This will help the users of the extracts of the Phyllanthus species studied to be sure of the compounds of the plants they use and at the same time guard against any toxicant that could be concealed in different parts of the medicinal plants so as to ascertain the safety of their consumption.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML