-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2014; 4(1): 8-21
doi:10.5923/j.ijmb.20140401.02
Pollen Micro-morphology of the Minuartia Species (Caryophyllaceae) in Iran
Golaleh Mostafavi 1, Iraj Mehregan 2
1Department of Biology, College of Basic Sciences, Yadegar-e-Imam, Khomeini (RAH) Branch, Islamic Azad University, Tehran, Iran
2Department of Biology, Science and Research Branch, Islamic Azad University, Tehran-Iran
Correspondence to: Golaleh Mostafavi , Department of Biology, College of Basic Sciences, Yadegar-e-Imam, Khomeini (RAH) Branch, Islamic Azad University, Tehran, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The present study compared pollen micro-morphological characters among 20 Iranian Minuartia species. For this purpose, mature pollen grains taken from unopened flowers, were prepared, fixed and exhaustively investigated using Scanning Electron Microscopy (SEM). In order to perform the pollen micro-morphology of Minuartia, and to find its significance in taxonomy of the group, qualitative and quantitative variables related to the shape, size, ornamentations and pores were studied. Cluster and PCA analyses of qualitative and quantitative data were performed to demonstrate the pollen grain similarities among the species. According to our results, Minuartia species exhibit either sub-spherical or polyhedral pollen shapes. Pollen size also varies among different species. The longest polar axis length (P) belongs to Minuartia meyeri Bornm. (34.3±0.26µm) and the smallest one to M. montana L. (15.8±0.26µm). Pore ornamentations differ from prominent granular to slightly or distinctly sunken granular. The number of pores also varies considerably depending on species. It ranges from 10 (in M. meyeri and M. acuminata Turrill) to 24 (in M. subtilis Hand.-Mazz.) on two pollen hemispheres. The most reliable characters in this study were pore diameter (annulus included) (D), equatorial diameter (E), polar axis length (P), the distance between two pores (d), pollen outline, Pore diameter (annulus excluded) (R), annulus diameter (a), P/E ratio, Puncta diameter and Echini diameter respectively. Echini vs puncta (Ec:Pu) diameter ratio appeared to be crucial for the distinction of some closely related species such as M. sublineata Rech.f. and M. lineata (Boiss.) Bornm., as well as M. montana and M. sclerantha (Fisch & C. A. Mey.) Thell. Moreover, three out of the 20 species have D: d ratio (pore diameter: the distance between two pores ratio) only ≥ 1µm. According to our results, some differences in quantitative and qualitative palynological characters of similar species were observed that could be useful. Despite the diagnostic value of palynological data at the species rank, it was not useful to circumscribe any taxonomic group at the higher ranks.
Keywords: Caryophyllaceae, Iran, Micromorphology, Minuartia, Morphological similarity, Palynology, SEM micrographs
Cite this paper: Golaleh Mostafavi , Iraj Mehregan , Pollen Micro-morphology of the Minuartia Species (Caryophyllaceae) in Iran, International Journal of Modern Botany, Vol. 4 No. 1, 2014, pp. 8-21. doi: 10.5923/j.ijmb.20140401.02.
Article Outline
1. Introduction
- Caryophyllaceae Juss., is a cosmopolitan family with 86 genera and approximately 2200 species. Most of these genera occur in temperate regions of the northern hemisphere and comprise both annual and perennial herbaceous plants, sub shrubs, more rarely shrubs or small trees that are usually monocious and rarely diocious (Bittrich 1993).The genus Minuartia L. [(Caryophyllaceae, subfam. Minuartioideae (Alsinoideae), Tribe Alsineae according to Takhtajan 1997)], is one of the smallest genera within the family; however, it includes many taxonomically difficult species with unclear taxa delimitations. Minuartia spp., have an extensive distribution and are endemic to Asia, Europe and North America (Schischkin 1936; Halliday 1964; Mc Neill 1967; Meikle 1977; Kamari 1997). The genus is recognized via its ex-stipulate leaves, veined sepals, three valves in capsule, three styles and also nearly rounded petal apex (Mc Neill 1967; Rechinger 1988). According to Rechinger (1988) only six out of the 21 species are endemic to the Iranian plateau, among which two species i.e., M. aucheriana Bornm. and M. acuminata, are endemic to the Iran. Minuartia was introduced by Linnaeus (1753) with three species M. dichotoma, M. campestris and M. montana among which only the last one exists in Iran. Fourteen out of 35 species listed in Flora Orientalis currently exists in Iran (Boissier 1867). According to Rechinger (1988), it comprises 21 species in Iran in two different subgenera, i.e., Spergella (Fenzl) Mc Neill, (with one annual species i.e., M. picta Bornm. and Minuartia (with 20 species in six sections) in Iran. Considering morphological similarities among the Iranian species, their taxonomic positions had been controversial for a long time. For this purpose, complementary studies such as seed micro-morphology (Mostafavi et al. 2013), pollen micro-morphology, karyology, molecular phylogeny are necessary to support or confirm current taxonomical data. Fior et al. (2006) worked on six Minuartia species belonging to three different subgenera, i.e. Minuartia, Rhodalsine (J.Gay) McNeill, and Spergella. Among the species studied by them, only Minuartia picta, was in common with the Iranian species. This species showed closely relationships to some Arenaria species in the parsimony strict-consensus tree based on simultaneous analysis of ITS and matK sequences. Also, Harbaugh et al. (2010) confirmed that Minuartioideae is not monophyletic and they called some genera, such as Minuartia and Arenaria, polyphyletic based on chloroplast genome sequences. The results of Greenberg and Donoghue (2011), based on nuclear ribosomal ITS region and five chloroplast genes and intergenic spacers, with a few exceptions, support the tribal classification presented by Harbaugh et al. (2010). Greenberg and Donoghue (2011) worked on only 20 species of the genus (none of them except M. picta existed in Iran) and found that Minuartia species appear in tribes Sclerantheae (A.L. Juss.) DC., Sagineae Tanf., Sperguleae Dumort., and Eremogoneae Rabeler & W.L.Wagner. Therefore, they are not strictly monophyletic. Caryophyllaceae pollen morphology has been studied (Erdtman 1952; Chanda 1962; Mc Neill and Bassett 1974; Moore and Web 1978; Ghazanfar 1984; Bittrich 1993; Taia 1994; Yildiz 2001; Perveen and Qaiser 2006; Ataslar, Potogluerkara, and Tokur 2009; Külköylüoglu, Yildiz, and Minareci 2009; Eröz and Ataslar 2010; Yildiz, Çirpici, and Dadandi 2010; Yildiz et al. 2011). These studies revealed various pollen shapes for plants belonging to this family. Depending on species, pollen grain could be tricolpate, sub-oblate to sub-prolate, porate, pantocolpate, spherical or rounded polyhedral (Mc Neill and Bassett 1974). Tectum is mostly punctitegillate, sometimes anulopunctate, rarely reticulate (Silene L. spp. and Cerastium indicum Wight & Arnott), spinulate or microechinate. Pollen shape in genus Minuartia (Subgen. Rhodalsine (J. Gay) Mc Neill is trizonocolpate with rather thin exine layer and is completely different from other species belonging to Alsinoideae. All the species placed in this subfamily obviously have pantoporate pollen grains with thick exine layer (other Minuartia species is included). Pore number is variable in Alsinoideae (12-40) and Caryophylloideae (15-38) (Erdtman 1968; Bittrich 1993; Mc Neill and Bassett 1974). Therefore this character could be more useful for determining taxa. In the Caryophyllaceae family most of the species like Arenaria L., Minuartia, Dianthus L., Stellaria L., Gypsophila L. and so forth exhibit pantoporate pollen grains and spinulose-scabrate to punctate tectum (Bittrich 1993; Perween and Qaiser 2006). According to the recent Turkish study (Ataslar, Potogluerkara, and Tokur 2009), Gypsophila species have spheroidal and pantoporate pollen grains. The exine is tectate and displays granulate-microechinate ornamentations in most species. It has been shown that the most important characters in genera with pantoporate pollen grains like Minuartia, are pore diameter, presence or absence of annulus, pore diameter (annulus included), the distance between two pores, pollen size and finally surface ornamentations (Punt and Hoen 1995). Based on apertural type and exine surface ornamentations, some Minuartia species were placed in Moehringia trinervia (L.) Clairv. type and some of them fell into Minuartia rubra Mc Neill type (Punt and Hoen 1995). Among Minuartia species belonging to the Moehringia trinervia type only M. hybrida (Vill.) Schischk. exists in Iran. In this type, pollen shape is usually ellipsoidal and less frequently spheroidal. Pores are circular to slightly elliptic, medium in size, slightly or distinctly sunken, annulus is narrow, operculum a shield beset with small microechinate granules with different size. The distance between two pores is larger than or sometimes equal to the pore diameter, annulus included. Minuartia hybrida group (M. hybrida and M. verna (L.) Hiern) remains nevertheless a rare exception since the distance between two pores is always shorter or equal to the pore diameter (annulus included). The exine is thick to very thick and usually become distinctly thinner towards the pores. Its ornamentations are microechinate with distinct puncta. Echini are distinct and irregularly arranged all over the surface. Its size and density is variable but it is usually small and usually not decreasing in number towards the pores. Puncta is usually larger or sometimes smaller than echini. Columella varies in size and is usually crowded. The Moehringia trinervia type is characterized by its narrow annulus, a distinctly larger distance between two pores than the pore diameter, its ellipsoidal shape and finally the presence of an operculum consisting of a shield beset with granules. The pore diameter is often relatively small (2-4 µm but in some species up to 6.5 µm). The outer margin of the annulus is frequently irregular because of the attached columellae. In this type, the number of pore ranges from 12-30. The Minuartia rubra type is M. setacea (Thuill.) Hayek and M. rubra, both absent from Iran. In these species, pollen grains are often small (with about 22 to 38 µm in diameter), spheroidal, pantoporate with a variable pore number ranging from 11 to 13 (always less than 15) (Punt and Hoen 1995). The pores are either circular or elliptic in outline, medium in size, sunken, with narrow or medium size annulus. Its operculum is consisted of a number of granules. In this type columella is usually small, uniform in size, more or less circular and often densely crowded (rarely widely spaced). The thick exine is microechinate with small puncta (much smaller than echini) and shows a decreasing thickness toward the pore. Finally, the Minuartia rubra type is characterized by its few small pores (usually around 12), distinct polygonal outline, large distance between two pores and relatively thin exine (2.5-3.5 µm). The pollen type has some resemblances with the Moehringia trinervia type but differs in the outline and number of pores. Based on the latest morphological studies on the taxonomy of the Iranian species, seven sections are detected for Subgen. Minuartia., among which three sections i.e., sect. Sabulina, sect. Acutiflorae and sect. Minuartia, have very closely related species (Based on an unpublished data). Minuartia umbellulifera (Boiss. & Balansa) Mc Neill, M. juniperina Maire & Petitm., M. glandulosa (Boiss. & Huet.) Bornm., M. aucheriana, M. sublineata, M. lineata and finally M. sabalanica Assadi & Mostafavi are closely related taxa that are placed in sect. Acutiflorae. Also, due to many morphological similarities, species M. micrantha Schischk., M. hamata Mattf., M. meyeri, M. decipiens, M. sclerantha and M. montana are all placed in the separate section i.e., sect. Minuartia. Section sabulina is another section of the subgen. Minuartia with four species i.e., M. hybrida, M. mesogitana Hand.-Mazz., M. subtilis and M. urumiensis Mattf. These species are also very closely related ones taxonomically. In this study, pollen micro-morphology of 20 Minuartia species belonging to two subgenera is investigated for the purpose of identifying some closely related taxa morphologically. For this reason, some characters that were mentioned as important in the previous studies on pollen grains of the family were used.
2. Materials and Methods
- Materials used in this study are listed and their vouchers are mainly preserved at TARI (Table 1).
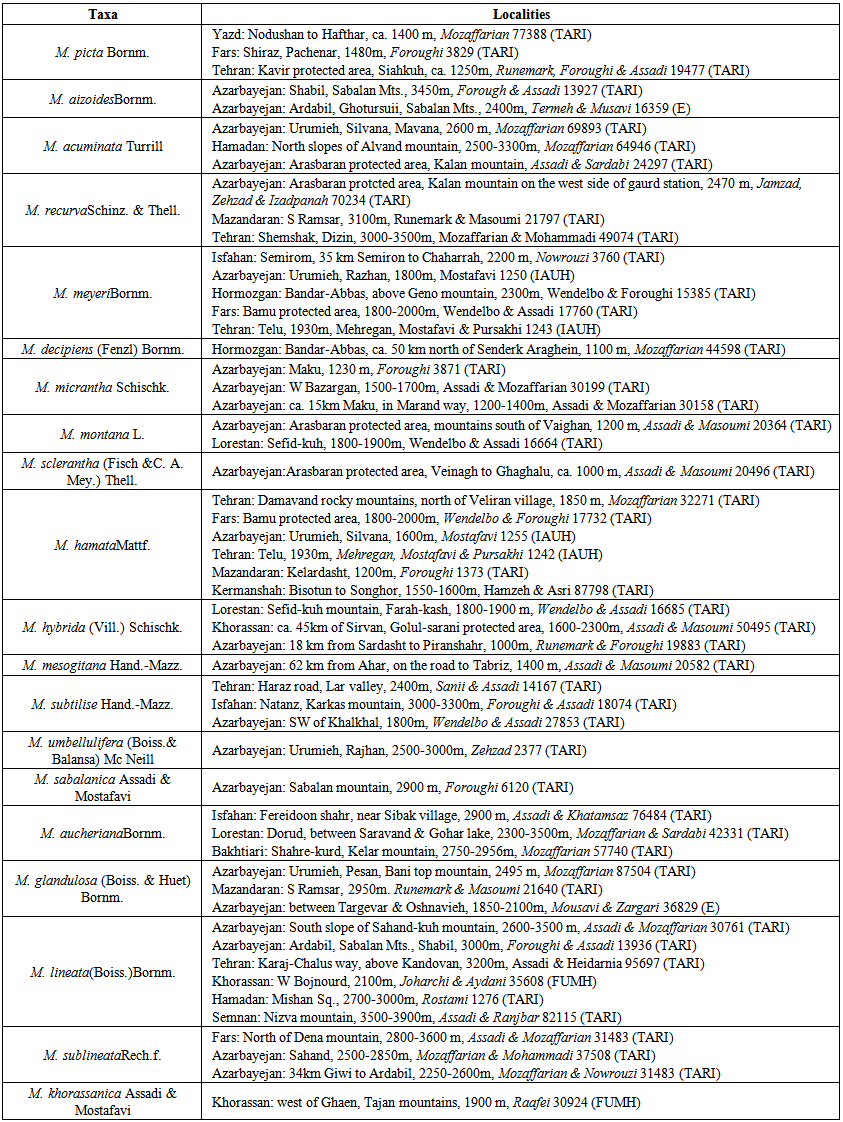 | Table 1. The list of the species used for pollen micro-morphological study and their localities |
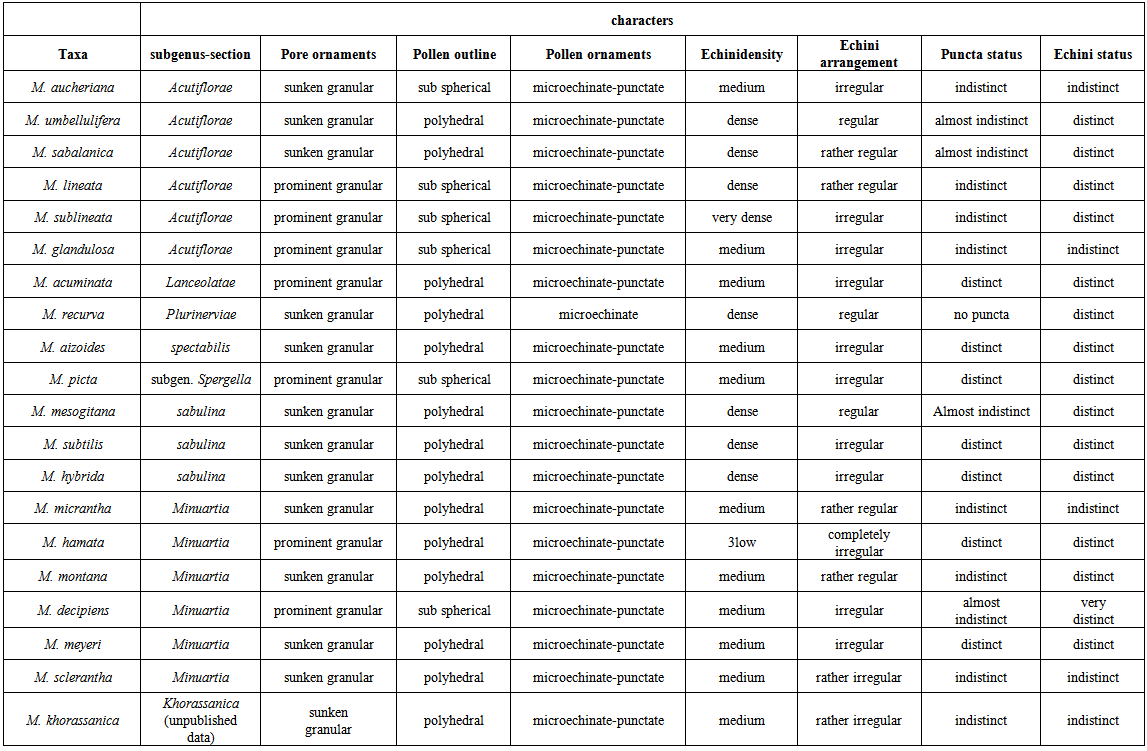 | Table 3. Comparison of qualitative pollen micro-morphological data in Minuartia sp |
3. Results
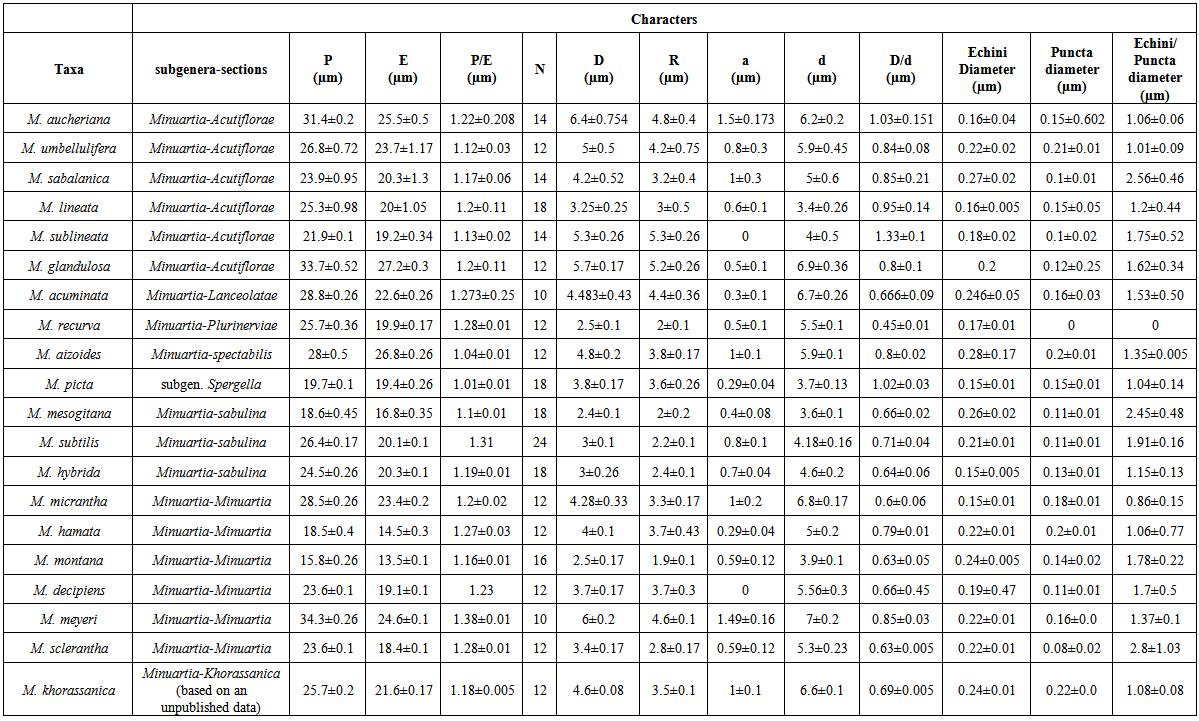
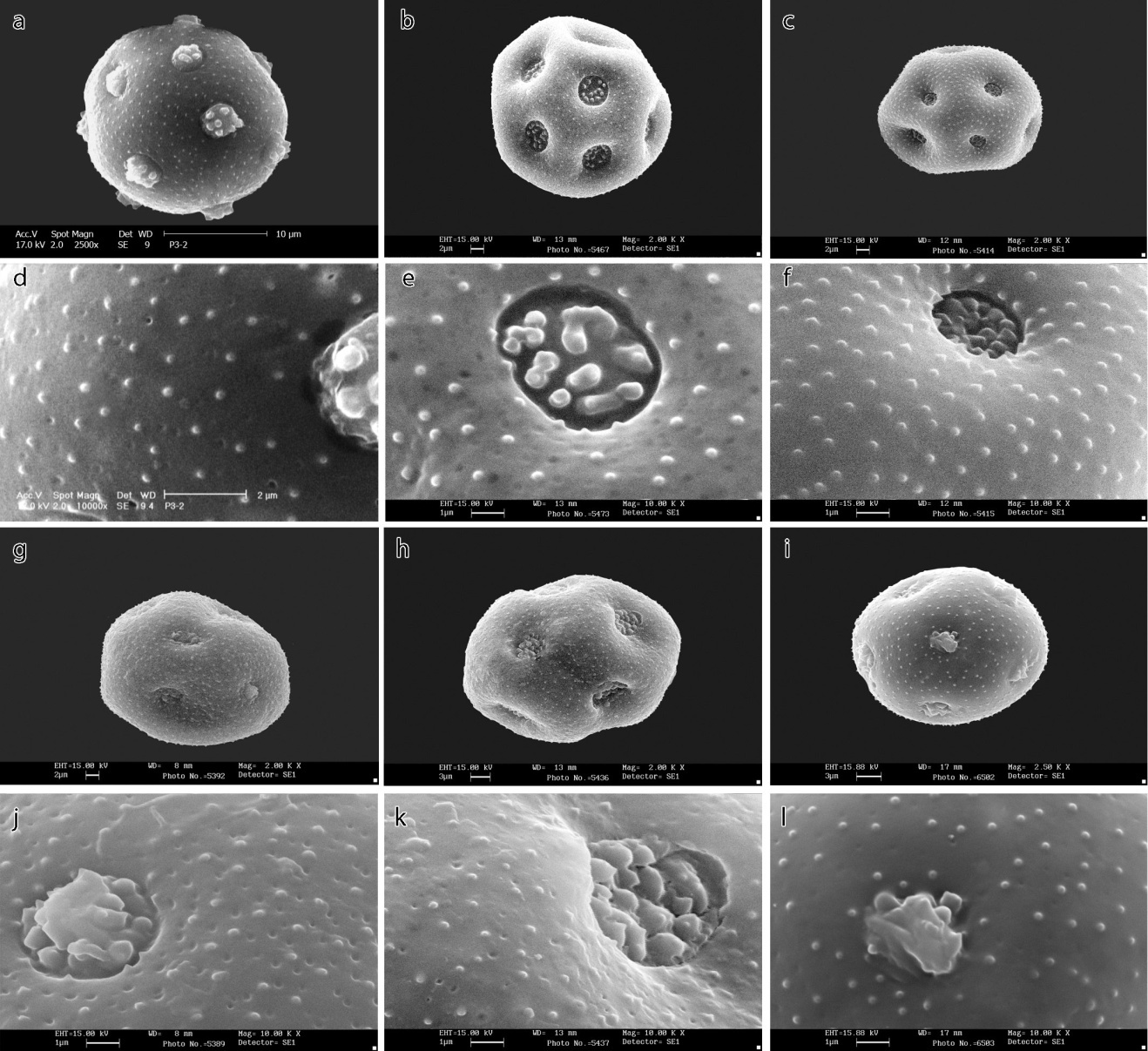
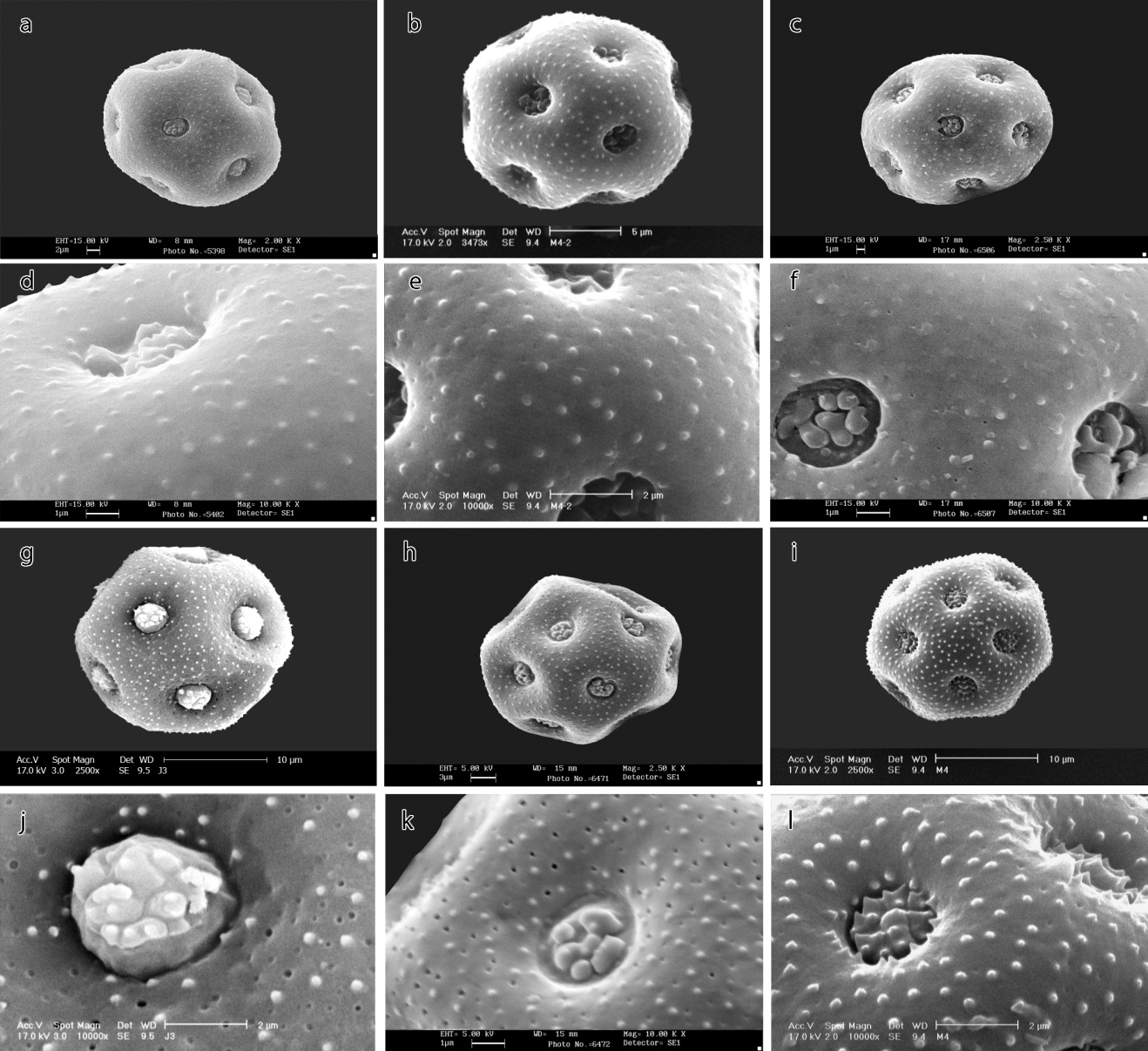
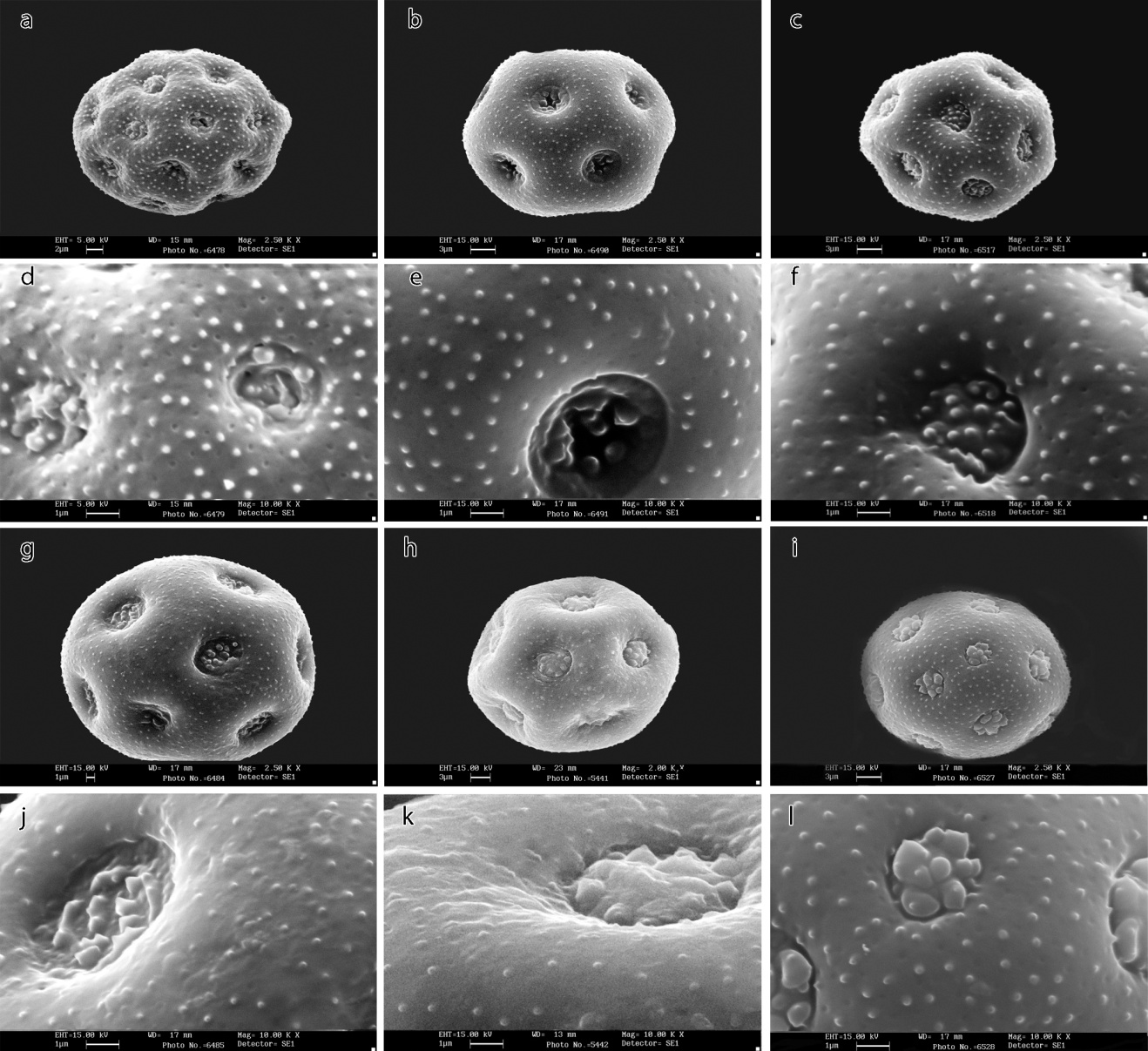
- Quantitative and qualitative characters are presented in Tables 2 and 3 (terminology according to Punt et al. 1994).
3.1. Quantitative Characters
3.1.1. Pollen Size
- Polar axis length (P), equatorial diameter (E) and P:E ratio were measured precisely. As shown in the table 2, the longest and smallest P were both devoted to annual species i.e., M. meyeri (34.3±0.26 µm) and M. montana (15.8±0.26 µm) respectively (Table 2). Pollen grains of the perennial Minuartia glandulosa (27.2±0.3 µm) and the annual M. montana (13.5±0.1 µm) had the longest and smallest E, respectively. P:E ratio was adjustable in all species from ~1 µm (M. picta and M. aizoides Bornm.) to 1.38±0.01 µm (M. meyeri).
3.1.2. Pore Size and Number
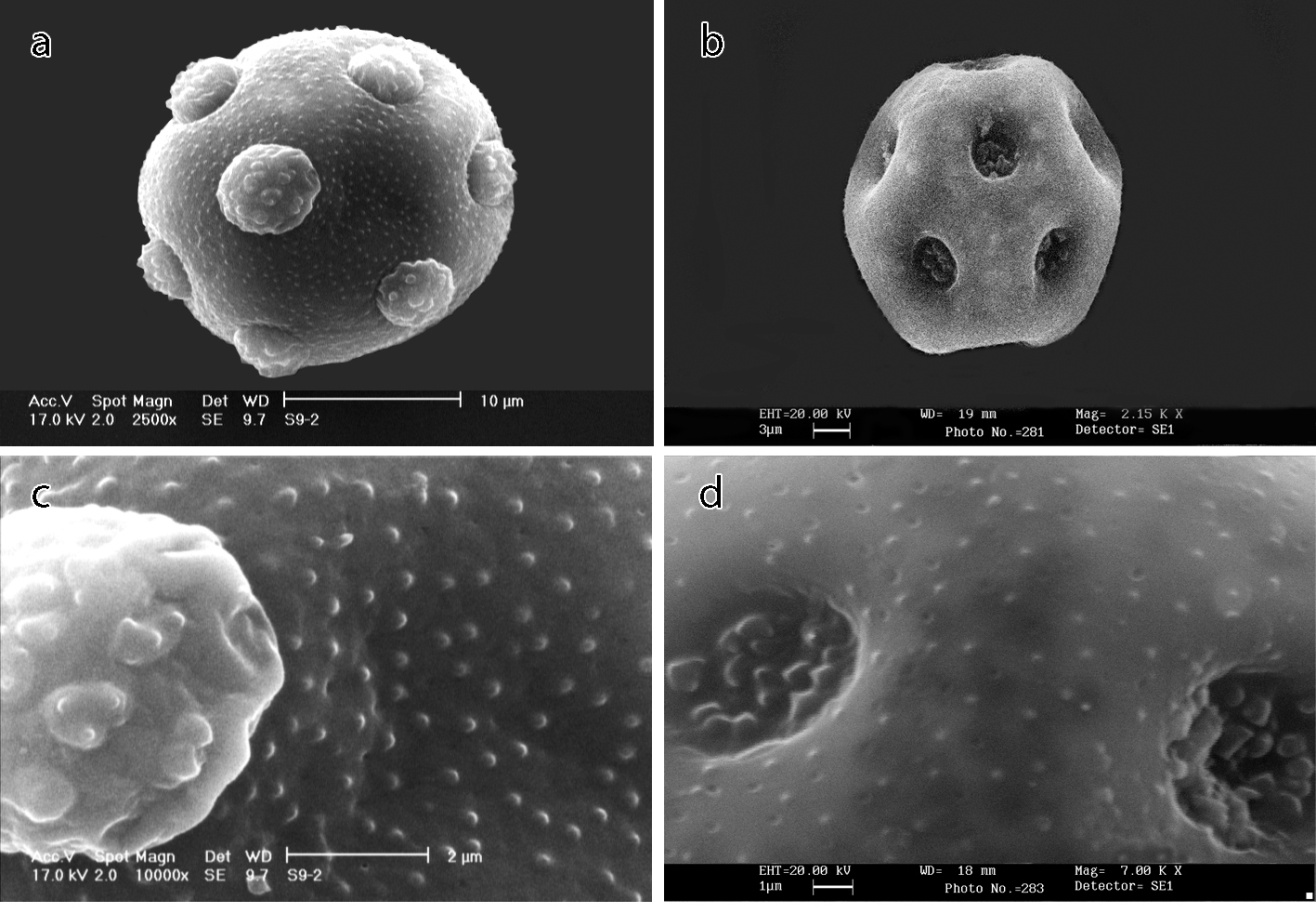
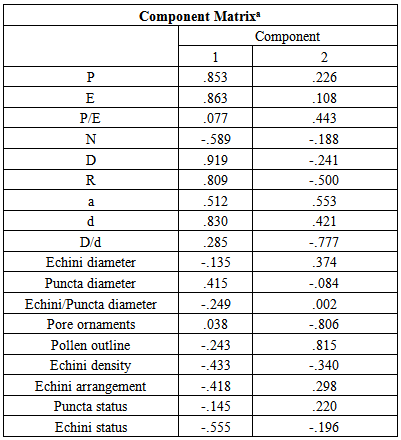
- Minuartia aucheriana (6.4±0.754 µm) and M. mesogitana, (2.4±0.1 µm) had longest and smallest pore diameter (annulus included) among the other taxa respectively. The largest annulus diameter belonged to M. meyeri and M. aucheriana and two species i.e., M. sublineata Rech. f. and M. decipiens had no annulus in around. The smallest distance between pores was observed in M. lineata (3.4±0.26 µm) while M. meyeri had the largest one (7±0.2 µm). The distance between two pores in M. aucheriana, M. lineata and M. sublineata was approximately equal or slightly shorter than pore diameter. In the other species this distance was longer than the pore diameter (Table 2).The number of pores was variable from five to 12 in one hemisphere therefore 10-24 pores are present in two hemispheres (counting based on Monoszon 1952). Minuartia subtilis had maximum pore number and M. meyeri as well as M. acuminata showed minimum pore number among the studied species. Overall, M. subtilis, M. mesogitana, M. hybrida, M. montana, M. lineata and finally M. picta pore numbers were 16 or more than 16. Others had 10 to 14 pores on two pollen hemispheres.The relation between pore diameter and pore distance obtained through our SEM analysis is shown (Figure 1).
 | Figure 1. Relation between pore diameter and pore distance |
3.1.3. The Size of Echinus and Punctum
- Echini to puncta diameter ratio (Ec:Pu) varied among taxa and therefore appeared to be a suitable character to differentiate similar species (Table 2). The lack of puncta was only observed in M. recurva Schinz & Thell. (sect. Plurinerviae) (Figure 2(f)). Minuartia sclerantha (sect. Minuartia) and M. micrantha (sect. Minuartia) had the largest and the smallest E:P ratio, respectively (Figure 3(f) Figure 3(e)). All other taxa as mentioned above, had microechinate-punctate ornamentations.
3.2. Qualitative Characters
3.2.1. Shape
- Two different shapes of pollen i.e., sub spherical and polyhedral could be found in the genus Minuartia. Sub-spherical pollen grains were observed in M. aucheriana, M. sublineata (Figure 5(a)), M. lineata (Figure 4(i)), M. glandulosa (Figure 4(h)) (sect. Acutiflorae), M. picta (subgen. Spergella) (Figure 2(a)) and M. decipiens (sect. Minuartia) (Figure 2(i)), while all other species had polyhedral pollen grains (Figures 2-5).
3.2.2. Ornamentations
- In almost all investigated species, pollen grain ornamentations were microechinate-punctate. As previously mentioned, only Minuartia recurva had no puncta on its pollen surface that could be a suitable character to distinguish it. In this species the operculum was ornamented with granules. Pores were slightly (approximately flattened) to distinctly sunken or completely prominent. Minuartia lineata, M. sublineata, M. glandulosa, M. acuminata, M. aizoides, M. hamata and M. decipiens exhibited a prominent granular type. All other species had an impressed granular form (Figures 2-5).
3.3. Cluster Analysis and PCA
- The results of cluster analysis and Principal Component Analysis for Minuartia species are shown in Tables 4 and 5. The cluster dendrogram demonstrates the species similarities. The phenoline drown at the top of each dendrogram, shows the taxonomical distances. High correlation coefficients indicate a high degree of similarity among taxa and therefore show low distance between them. In cluster analysis, the species firstly were divided into two major groups (Table 4). Minuartia aizoides, M. acuminata, M. khorassanica Assadi & Mostafavi, M. micrantha, M. umbellulifera, M. meyeri, M. glandulosa and M. aucheriana were classified as one major group (i.e., group I) and other 12 species were included in the group II. Between 0-5 taxonomical distances, the group I, was divided into two secondary groups. The species Minuartia meyeri, M. aucheriana and M. glandulosa were placed in the group A and other mentioned above species, were categorized in the group B of the secondary group. Also, in 5-10 taxonomical distances, group II was divided into two secondary groups. The group A was consisted of M. montana, M. hamata, M. mesogitana and M. picta. The group B was also divided into two secondary groups in taxonomical distance of 5. The species M. subtilis, M. hubrida and M. lineata were placed in the separate group and M. sublineata, M. recurva, M. sabalanica, M. sclerantha and finally M. decipiens were placed in juxtaposition in the other secondary group (Table 4).
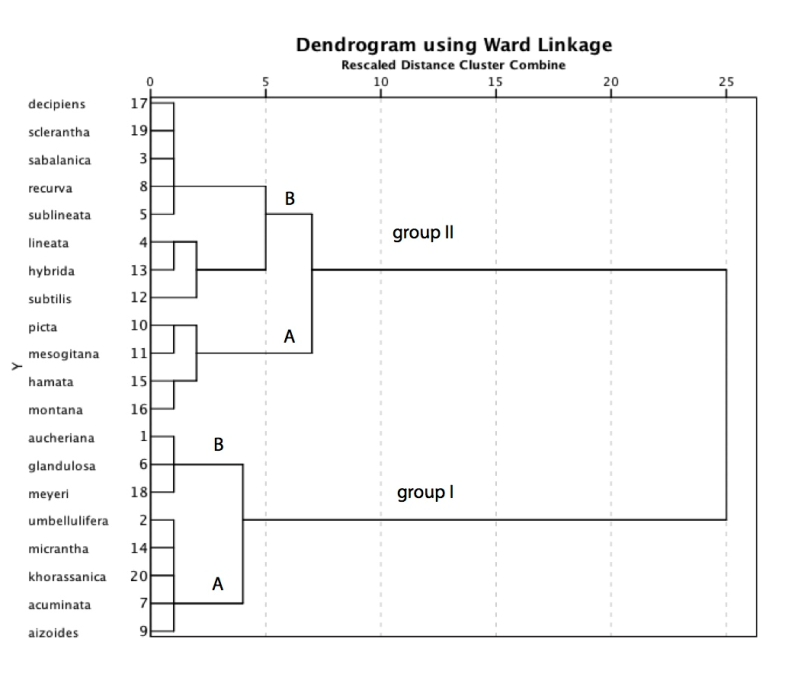 | Table 4. Cluster dendrogram showing species similarities based on pollen micro-morphological data |
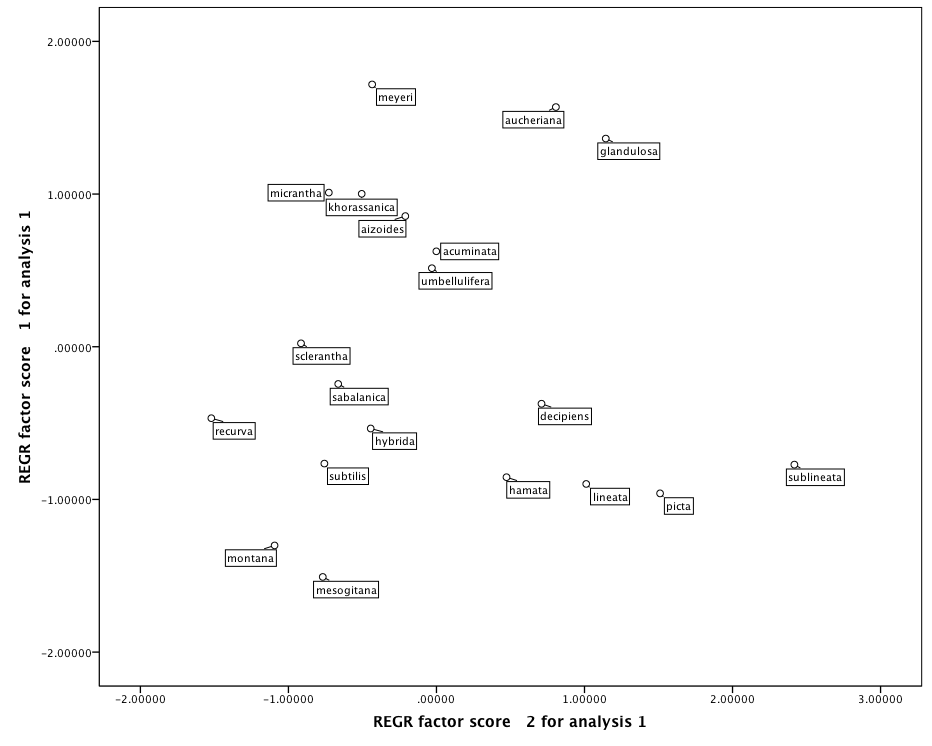 | Table 5. PCA, showing similarities among taxa in two dimensions based on pollen micro-morphological data |
|
4. Discussion
- According to Punt and Hoen (1995) two types of pollen grains were identified for Minuartia species considered in that article.These two types are characterized by some major characters such as the distance between two pores, annulus thickness, exine thickness, pore number, pollen outline, the number of granules in operculum region and also pore diameter.According to our results in the present study, these two types are not sufficient to classify all Minuartia species. In some cases, the species display mixed characters and therefore, could not be placed in either Minuartia rubra or Moehringia trinervia type. Some of the most important characters (with the variance of > 0.8) in the present study were pore diameter (annulus included), equatorial diameter, polar axis length, the distance between two pores and pollen outline. The results of cluster analysis and Principal Component Analysis showed that despite separation of some closely related taxa via pollen micro-morphological data, no distinct group is detected that could conform the correlation among taxonomic groups in the section and subgenus ranks. In other words, it could only separate similar taxa at the species level. Here we compare some representatives of each section of Minuartia placed in subgen. Minuartia and the only species in sect. Spergella separately.At first, the results obtained for six species belonging to sect. Acutiflorae i.e. M. aucheriana, M. lineata, M. sublineata, M. umbellulifera (Boiss.) Mc Neill, M. sabalanica and M. glandulosa are discussed:Minuartia sabalanica, a new Iranian species (sect. Acutiflorae), (Mostafavi et al. 2011), showed a higher Ec: Pu diameter ratio compared to other species belonging to the section (2.56±0.46 µm) (Table 2). This ratio could separate this new species from its closely related species in section i.e., M. umbellulifera (1.01±0.09). It reflected the fact that echina is much larger than puncta in M. sabalanica. Despite the presence of quite smaller puncta in this newly identified species, D:d and P:E ratios did not show considerable differences compared to M. umbellulifera. Moreover, the difference in pore number on two pollen hemispheres between M. umbellulifera (12 pores) and M. sabalanica (14 pores) was also significant. Echinus density was the same in both species (Tables 2, 3). Minuartia lineata and M. sublineata belonging to sect. Acutiflorae are distinguishable using only a few characters such as plant color and pedicel length. Our results illustrated that these species could also be dissociated through pollen micro-morphological characters. The great majority of species belonging to the sect. Acutiflorae except for M. aucheriana and M. sublineata, have a D:d ratio less than 1µm. It means that the distance between two pores is larger than the pore diameter, annulus included. In M. aucheriana and M. sublineata this ratio was between 1-1.33 µm. The largest polar axis length in this section was observed in M. glandulosa and the smallest one in M. sublineata. Minuartia aucheriana, M. sabalanica and M. umbellulifera represented species displaying pores with slightly to distinctly sunken granular ornamentations while the other three species had prominent granular pores. Among species belonging to the sect. Acutiflorae, only M. sabalanica and M. umbellulifera had a polygonal outline. This character obviously proves their taxonomical relationships. The next section including six Iranian species is sect. Minuartia: Minuartia meyeri showing important morphological similarities with M. decipiens subsp. persica. This fact explains their juxtaposition with each other. However, they have some morphological differences in their pedicel length and sepal base shape. The latter subspecies is endemic to the country and is reported from Southern Iran. My palynological investigations showed some obvious additional differences. According to the results, pollen grains of the two closely related species i.e., M. decipiens and M. meyeri have considerable differentiating characters and could be easily dissociated via pollen micro-morphology. This, reflects the fact that we could not disregard the role of pollen morphology in distinguishing some species that have many morphological similarities.Minuartia montana and M. sclerantha not only have many morphological resemblances but also have the same distribution (NW Iran). The results of this study revealed some differences in palynological characters such as E:P diameter ratio, echini arrangement, pollen size and also the number of pores. It should be mentioned that the Ec:Pu diameter ratio in M. sclerantha is 2.8±1.03µm, which is more than the other species in the genus. Puncta size in M. sclerantha was very short (0.08±0.02 µm) but its puncta approximately was as large as M. montana. Pollen size in M. sclerantha was more than M. montana while the number of pores in M. montana was more than what observed in M. sclerantha. Other quantitative data like D:d ratio, P:E ratio and so on were not so variable. Echini in M. sclerantha was distinct and rather regularly arranged throughout the surface but in M. montana this status was inversed. The cluster dendrogram shows these differences via putting them in two separate secondary clusters A and B of the group II. Another species, that falls into sect. Minuartia, is Minuartia hamata showing different morphological features in comparison to other species placed in this section. This species showed a few differences in pollen morphology such as its echini density. Echinus is distinct and is arranged irregularly with low density. It should be mentioned that the results of seed micro-morphology for the determination of its taxonomic position among the other species as well as pollen results was sine qua non not per se adequate (Mostafavi et al. 2011). More evidences such as molecular phylogeny could be effective to designate precisely its taxonomic position. The results of cluster analysis and PCA demonstrated that this species is near to the related species i.e., M. montana (cluster A of the group II) that are both placed in the sect. Minuartia. Other species belong to the mentioned above section, are scattered in different clusters. Four species i.e., M. mesogitana, M. hybrida, M. subtilis and M. urumiensis is placed in sect. Sabulina according to Rechinger (1988). In this study, we investigated only three of them. Minuartia hybrida, M. mesogitana and M. subtilis had many morphological resemblances making their taxonomical dissociation difficult. The results of palynological studies showed that M. subtilis could be separated from the two other species (24 pores in two hemispheres) because of its higher number of pores. However, Minuartia mesogitana and M. hybrida could not be dissociated via this character as they have exactly the same pore number. Minuartia subtilis was discernible from M. hybrida and M. mesogitana by its longest P:E ratio (1.31 µm). However, in this case, characters such as pollen shape and pore ornamentations were not such reliable (Table 2). Undeniably, one of the most important characters for detecting M. mesogitana was a higher E: P ratio compared to two other species. Moreover this pollen appeared to be smaller than those released from M. hybrida and M. subtilis. Separation of Minuartia mesogitana from two remained taxa is demonstrated in Table 4. It is placed in the cluster A of the major group II, while two remained species were in juxtaposition in a secondary group of the cluster B. Minuartia khorassanica (Mostafavi et al. 2011b) was another new species showing polyhedral pollen grains and sunken granular pore ornamentations. Therefore this species considerably differs with the nearest one, M. lineata releasing sub spherical pollen grains with different pore ornamentations. Moreover pore numbers and annulus diameter was also different despite the same Ec: Pu ratio. Cluster dendrogram confirmed above statements about the separation of these two nearest taxa. Minuartia recurva was the only species that could be easily differentiated from other Minuartia species by having no puncta on its pollen surface. The results of the cluster analysis could not separate it from all other taxa.This is quite considerable that only in two distinguishable taxa, M. aizoides and M. picta, from two different subgenera (i.e., Spergella and Minuartia (sect. Spectabilis)), P:E ratio was equal to 1. Although the results of cluster analysis and PCA could not show taxonomic similarities among the species placed in a certain group, it could be useful in determining some closely related taxa. Considering these statements we reach a conclusion that pollen micro-morphological studies have had considerable role in distinguishing some related taxa at the species rank. In the near future, karyological studies and molecular investigations will be also performed in order to confirm the taxonomic position of the Minuartia spp. growing in Iran.
ACKNOWLEDGEMENTS
- The authors wish to thank Dr. Y. Shahali for his helps in editing the manuscript and also Mrs. S. Eshghi and Mr. A. Rezaie for preparing SEM micrographs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML