-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2014; 4(1): 1-7
doi:10.5923/j.ijmb.20140401.01
Fruit Flavonoids of Some Species of Subgenera Esula and Chamaesyce (Euphorbia) in Iran
1Young Researchers Club, Arak Branch, Islamic Azad University, Arak, Iran
2Department of Biology, Faculty of Science, Arak University, 38156-8-8349, Arak, Iran
Correspondence to: Mitra Noori, Department of Biology, Faculty of Science, Arak University, 38156-8-8349, Arak, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this research, fruit flavonoids characters of some collected Euphorbia species (subgenera Esula and Chamaesyce) from Markazi province, Iran area were studied using 2-Dimentional Paper Chromatography (2-DPC), Thin Layer Chromatography (TLC) and available standard flavonoids. Voucher specimens were prepared for reference as herbarium voucher. Our studies showed that all of studied Euphorbia species had flavonoids considerably. Flavonoid sulphates and flavone C- and C-/O-glucosides existed in fruit of studied taxa, while dihyro flavonol 3-O-monoglycosides just were found in E. falcata. Kaempferol were detected in all of studied species in contrast to rhamnetin which was found only in E. ozyridiforma. Concenteration and variety of flavonoid componds in studied taxa of subgenera Esula were more than subgenera Chamaesyce. Some flavonoid compounds including myricetin, naringenin and rhamnetin were not detected in Euphorbia studied species of subgenera Chamaesyce.
Keywords: Subgenera Esula, Subgenera Chamaesyce, Euphorbia, Flavonoids compounds, Chromatography
Cite this paper: Mahdi Kaveh, Mitra Noori, Fruit Flavonoids of Some Species of Subgenera Esula and Chamaesyce (Euphorbia) in Iran, International Journal of Modern Botany, Vol. 4 No. 1, 2014, pp. 1-7. doi: 10.5923/j.ijmb.20140401.01.
Article Outline
1. Introduction
- The Euphorbiaceae is one of the larger families of flowering plants with ca. 300 genera and 8000 species [1, 2]. The genus Euphorbia belongs to the subfamily Euphorbioideae and comprises ca. 2000 species, mainly distributed in subtropical and warm temperate regions [3, 4]. Euphorbia subgenus Esula is the largest subgenus within Euphorbia, which largely corresponds to Boissier's Euphorbia sect. Tithymalus and has about 500 species. Subgenera Chamasyce is the large segregate genus from Euphorbia with about 300 species. Euphorbieae and Euphorbia are generally considered taxonomically difficult, and a considerable degree of uncertainty has always existed about the relationships of the groups within them [5]. Plant chemosystematics is the application of chemical data to systematic problems. It is a rapidly expanding interdisciplinary field concerned with using chemical constituents for explaining relationships between plants and inferring phylogeny [6]. Flavonoids are popular compounds for chemotaxonomic surveys of plant genera and families because of their almost ubiquitous presence in vascular plants, structural variety, ease of detection and relative ease of identification [7]. They are the most numerous of the phenolics and are found throughout the plant kingdom [8]. To date, more than 6400 different flavonoid compounds have been identified [9] and the pathways responsible for their synthesis have been characterized in detail in numerous plant species [10, 11, 12, 13, 14]. Flavonoids are present in high concentrations in the epidermis of leaves and the skin of fruits and have important and varied roles as secondary metabolites. They are widely distributed in foods and beverages of plant origin, such as fruits, vegetables, tea, cocoa, and wine [15, 16]. Within the subgroups of the flavonols and the flavones, the flavonol quercetin is the most frequently occurring compound in foods and also kaempferol, myricetin, and the flavones apigenin and luteolin are common [17]. Different classes of flavonoids and their conjugates have numerous functions during the interactions of plant with the environment, both in biotic and abiotic stress conditions [18, 19]. Many flavonoids are active principles of medicinal plants and exhibit pharmacological effects [20]. Flavonoids play a variety of significant functions in plants as signal molecules [21], agents for pollen germination [22, 23, 24, 25, 26, 27], and seed germination [28, 29], pollinator attractants [30], regulators of auxin transport [18, 31, 32, 33], UV filters [34, 35], antimicrobial [36, 37] and antiherbivory agents [38]. Some flavonoids such as quercetin, kaempferol, myricetin, apigenin, and leuteolin also have antioxidative activity in many in vitro studies [39]. Moreover, plants use the huge variety of secondary metabolites as tools to overcome stress [40]. Flavonoids are a diverse group of natural product found in all plants [41, 42]. Bryophytes (mosses), liverworts, hornworts are the oldest plant group to produce chalcone, flavonols, and flavones among the types of flavonoids [43]. Leguminosae produce about 28% of all known flavonoids, 95% of all isoflavonoid aglycones and about 850 compounds, including 362 isoflavones which were known in this family [44, 45] .Thirty-three kinds of flavonoids were reported from Polygonaceae species leaves [46]. Flavonoid compounds were found widely in Euphorbiaceae and there are some studies in this connection. Noori et al (2012) showed that all studied populations of Chrozophora tinctoria and C. hierosolymitana in Markazi Province contain flavonoid sulphates, flavone C and C-/O-glycosides and aglycon. Also all studied populations have apigenin and quercetin, while rutin was just found in 4 populations of C. tinctoria species [47]. Five flavonoid glycosides were reported in the methanol extract of the aerial parts of Chrozophora tinctoria [48]. Four flavonoids were isolated from the butanolic extract of the aerial parts of Croton campestris St. Hill. (Euphorbiaceae) [49]. Eleven flavonoid compounds -one C-glycosyl flavone and ten flavonol glycosides- were isolated and identified from leaf material of Cnidoscolus aconitifolius, C. souzae, and C. spinosus [50]. Several studies by different researchers indicated that flavonoids occurred widely in various species of Euphorbia [41, 42, 51-72]. The aim of this study is to describe fruit flavonoids of 18 Euphorbia species from Iran in two subgenera Chamaesyce and Esula.
2. Materials and Methods
2.1. Sampling and Identification
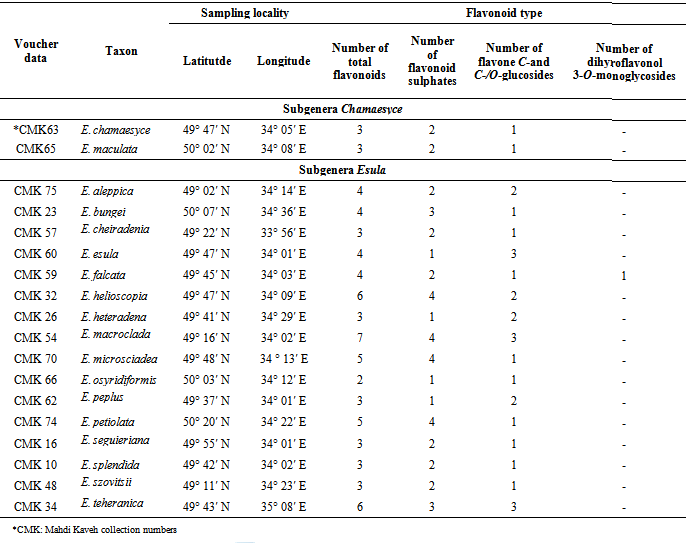
- Mature fresh fruits of 18 Euphorbia species were collected from some area, described in Table 1. Plants identified using available references [73, 74, 75]. Samples were air dried for detection and identification of flavonoids.
2.2 Extraction of the Plant Material
- For a comparative analysis of the flavonoids, small extracts of all the accessions were prepared by boiling 200 mg of powdered air dried fruit material for 2 min in 5 ml of 70% EtOH. The mixture was cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40°, and taken up in 2 ml of 80% MeOH for analysis by 2-Dimensional Paper Chromatography (2-D PC).
2.3. Flavonoid analysis by 2-Dimensional Paper Chromatography (2-DPC)
- For the detection of flavonoids, ca 20 μl of each of the small extracts was applied to the corner of a quarter sheet of Whatman No 1 chromatography paper as a concentrated spot (10 applications of 2μl). The chromatogram for each sample was developed in BAW (n-BuOH-HOAc-H2O=4:1:5; V/V; upper layer), 1st direction, and HOAc (=15% aqueous acetic acid), 2nd direction, with rutin (= quercetin 3-O-rutinoside) as a standard. After development, the chromatograms were viewed in longwave UV light (366 nm) and any dark absorbing and fluorescent spots were marked. Rf -values in BAW and 15% HOAc were calculated.
2.4. Methods of Identification of the Flavonoids
- After obtaining sufficient amounts of purified flavonoids, as in the case of the flavonoids from 18 Euphorbia species, they were identified by means of UV spectroscopy using shift reagents to investigate the substitution patterns of the flavonoids [76, 77] and by acid hydrolysis to identify the aglycone and sugar moieties. Cochromatography with standards was also performed where possible. Flavonoid standards available for comparison during the study were rutin, kaempferol, quercetin, myricetin, naringenin, apigenin, luteolin, and rhamnetin (all obtained commercially, rutin from Merck and the rest from Fluka).
2.5. Acid Hydrolysis and Identification of Flavonoid Aglycones
- A small amount of each purified flavonoid (ca 0.5 mg) was dissolved in 0.5 ml of 80% MeOH in a test tube. To this sample 2 ml of 2M HCl were added and the mixture was heated in a water bath at 100C for 0.5 h. The solution was cooled, 2 ml of EtOAc were added and thoroughly mixed with the aqueous layer using a whirley mixer. The upper EtOAc layer was removed with a pipette, evaporated to dryness, dissolved in 0.5 ml of MeOH and applied as spots on thin layer chromatograms=TLC (cellulose). The TLC plates were run in three solvents alongside standards to identify the aglycone moiety [78].
3. Results
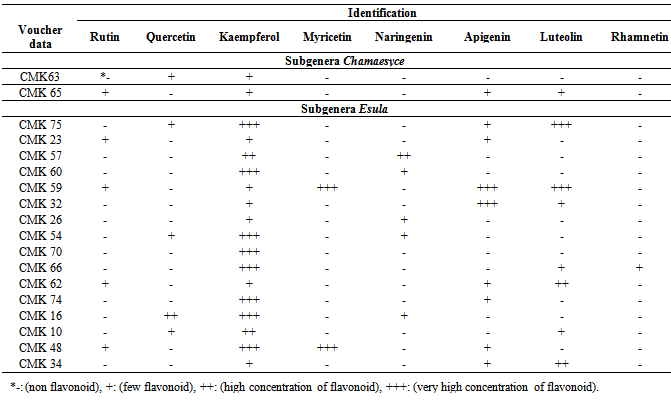
- All studied Euphorbia species contained flavonoid compounds in their fruits. Data in Table 1 and 2 show the collection information and also two-dimentional paper and thin layer chromatographical data of 18 studied Euphorbia species in two subgenera Esula and Chamaesyce from central of Iran. Table 1 shows 2-DPC and Table 2 show TLC data of studied Euphorbia species in two subgenera Esula and Chamaesyce from Markazi Province, Iran. The results showed existing flavonoid sulphates and flavone C-and C-/O-glucosides in all of studied taxa fruits but dihyro flavonol 3-O-monoglycosides were not found in them with the exception of E. Falcata (Table1 1). The most flavonoids number was observed in E. macroclada species fruits and E. osyridiformis showed the lowest (Table1 1). Kaempferol was found in all of studied species fruits. While rhamnetin was detected only in E. osyridiformis and myercetin existed in E. falcata and E. szovitsii (Table 2). All of studied samples had not quercetin with the exception of 5 species (E. chamaesyce, E. macroclada, E. seguieriana, E. splendida and E. aleppica). For other flavonoids is refered to Table 2. Our investigation into studied Euphorbia species illustrated that the variety and concentration of flavonoid compounds in studied Euphorbia species in subgenera Esula is more than subgenera Chamaesyce (Table 1 and Table 2).
|
|
4. Discussion and Conclusions
- As table 1 shows, flavonoid sulphates and flavone C-and C-/O-glucosides were detected in fruit of studied taxa, but dihyro flavonol-3-O-monoglycosides were not found in studied taxa except E. falcata (Table 1). Flavonoid solphates and flavone C-and C-/O-glucosides were reported in leaf of studied Euphorbia species [41]. Kaempferol was predominant flavonoid in extracts of studied taxa (Table 2). Quercetin, and kaempferol were reported as the most representative compounds for the genus Euphorbia [41]. Kaempferol was reported in E. helioscopia [55], E. hirta [69], E. chamaesyce, E. cordifolia, E. osyridiformis, E. heteradena, E. bungei, E. peplus, E. esula, E. falcata, E. szovitsii, and E. seguieriana [41]. Kaempferol 3-O-glucoside and quercetin 3-O-glucoside were obtained from E. larica, E. virgata, E. chamaesyce, and E. magalanta [61]. Papp et al (2005) studies showed that arial parts of Euphorbia cyparissias had 2 main flavonoids: kamfpherol-3- glucuronide and quercetin-3-glucuronide [68]. The hypotensive principales of E. maddeni were found to be kaempferol-4'-O-glucose and hyperin [57]. Result showed that quercetin was identified in E. macroclada, E. seguieriana, E. splendida, E. Chamaesyc and E. aleppica among studied species (Table 2). Quercetin was found in Euphorbia pilulifera [52], E. helioscopia [55], E. hirta [69, 71], E. chamaesyce, E. ozyridiforma, E. heteradena, E. bungei, E. helioscopia, E. petiolata, E. peplus, E. esula, E. falcata, E. szovitsii, E. microsciadea, E. cheiradenia, E. macroclada, E. seguieriana, E. splendida [41] and Euphorbia wallichii [42]. Some known derivatives of quercetin were isolated from different Euphorbia species by researchers [58, 60, 62, 67, 68, 72]. In this study, rutin was detected in Euphorbia bungei, E. peplus, E. falcata, E. szovitsii and E. maculata (Table 2). Rutin was identified in E. larica, E. virgata, E. magalanta [61], and leaves extracts of E. chamaesyce, E. cordifolia, E. ozyridiforma, E. heteradena, E. bungei, E. helioscopia, E. peplus, E. esula, E. falcata, E. szovitsii, E. teheranica, E. microsciadea, E. cheiradenia, E. seguieriana, E. splendida [41] and E. hirta [71]. As table 2 shows, Euphorbia falcata and E. szovitsii among studied species just have myricetin. Myricetin was reported in leaves extract of E. macroclada and E. petiolata [41] and in E. hirta [71]. Murillo and Jakupovic (1998) identified myricetin-3- rhamnoside and one flavonoid glycosides in E. aucherii which was collected in Iran [65]. Ghanadian et al (2012) identified myricetin 3-O-β-D-galactopyranoside in aerial parts of Euphorbia microsciadia [72]. Result shows that there are luteolin in some studied species, including E. ozyridiforma, E. helioscopia, E. peplus, E. teheranica, E. falcata, E. splendida, E. aleppica and E. maculata (Table 2). Omurkhamzinova and Erzhanova (1985) identified luteolin-3-rhamnoside and luteoline-3-galactoside from the aerial parts of E. soongarica and E. alatavica [59]. Our study indicated that rhamnetin was absent in all studied taxa except E. ozyridiforma (Table 2). Muller and Pohl (1970) isolated six new flavonoids all being glycosides of rhamnetin from E. amygdaloides [54]. Apigenin was detected in this study in E. maculata, E. bungei, E. helioscopia, E. petiolata, E. peplus, E. falcata, E. szovitsii, E. teheranica, and E. aleppica (Table 2). Apigenin was characterized in nineteen Euphorbia species [79]. The major flavonoids of Euphorbia prostrata are apigenin, luteolin, apigenin-7-glucoside and luteolin-7-glucoside [63]. Five apigenin glycosides were identified in E. humifusa [80]. As table 2 shows, naringenin were detected in Euphorbia heteradena, E. esula, E. cheiradenia, E. macroclada and E. sequieriana. Roshchin et al. (1970) found naringenin 7-O-β-D glucofuranoside for the first time in genus Euphorbia [81]. Myricetin, naringenin, and rhamnetin were not detected in fruits extracts of Euphorbia maculata and E. chamaesyce of subgenera Chamaesyce (Table 2). Finally, this study showed that the diversity of flavonoids in studied taxa is considerable and the concentration and variety of flavonoid compounds in studied species in subgenera Esula is more than studied taxa in subgenera Chamaesyce. It is believed that the high variety of flavonoid compounds in studied Euphorbia species is to their response to environmental stresses because all of studied taxa were weed and grow in destroyed pasture. Flavonoids protect plants against various biotic and abiotic stresses and play an important role in the interaction between the plant and their environment [13, 39, 82, 83]. However, further work is needed for attaining an obvious mechanism of flavonoid roles against stress protection.
ACKNOWLEDGEMENTS
- The authors would like to thank of Organic Chemistry Laboratory Experts, Chemistry Department in University of Arak.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML