-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2013; 3(2): 20-24
doi:10.5923/j.ijmb.20130302.02
Mutagenic Effects of Hydroxylamine and Hydroquinone on some Agronomic and Yield Characters of Soyabean (Glycine max (L.) Merr.)
J. K. Mensah, G. O. Okooboh, I. P. Osagie
Department of Botany, Faculty of Natural Sciences, Ambrose Alli University, Ekpoma, Nigeria
Correspondence to: J. K. Mensah, Department of Botany, Faculty of Natural Sciences, Ambrose Alli University, Ekpoma, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Dry and healthy seeds of soyabean (Glycine max (L.) Merr.) of about 10% moisture content were exposed to varying concentrations of 0-0.300% of Hydroxylamine and Hydroquinone for 24hours and their effects on germination, survival, chlorophyll content, agronomic and yield characters reported. Soyabean responded differentially to the chemicals for the parameters studied. The useful traits observed in the present work for both chemicals include increase in plant height/number of branches, number of flowers per plant, increase in the number of pods per plant. The two chemicals also increased the chlorophyll content and induced early maturity. However, these useful traits identified during the present study need to be tested further on a wider scale in later generations in order to isolate specific mutants with improved characters.
Keywords: Mutagenic Effects, Hydroxylamine, Hydroquinone, Yield Characters
Cite this paper: J. K. Mensah, G. O. Okooboh, I. P. Osagie, Mutagenic Effects of Hydroxylamine and Hydroquinone on some Agronomic and Yield Characters of Soyabean (Glycine max (L.) Merr.), International Journal of Modern Botany, Vol. 3 No. 2, 2013, pp. 20-24. doi: 10.5923/j.ijmb.20130302.02.
Article Outline
1. Introduction
- The mutagenic property of mutagens was first demonstrated in 1927, when Hermann Muller discovered that X-rays can cause genetic mutation in fruit flies, producing phenotypic mutants as well as observable changes to the chromosomes[1]. Similar work by Lewis Stadler also showed the mutational effect of X-rays on barley in 1928[2] and ultraviolet (UV) radiation on maize in 1936. Mutation breeding has been widely used for the improvement of plant characters in various crops. It is a powerful and effective tool in the hands of plant breeders especially for autogamous crops having narrow genetic base[3]. The prime strategy in mutation breeding has been to upgrade the well adapted plant varieties by altering one or two major agronomic material traits which limit their productivity or enhance their quality. Mutation induction offers significant increase in crop production[4] and the possibility of inducing desired attributes that either cannot be found in nature or have been lost during evaluation. Treatment with mutagens alters genes or break chromosomes. Induced mutations have been used to improve a wide variety of crops with different reproductive systems and at different ploidy levels[5].Chemical mutagens were not demonstrated to cause mutation until the 1940s, when Charlotte Auerbach and J.M. Robson found that mustard gas can cause mutations in fruit flies[6]. A wide range of chemicals have also been identified and tested for their mutagenicity in biological systems. These include nitrous acid, the alkylating agents, hydroxylamine, hydroquinone, sodium azide and the antibiotics–streptomycin, chloramphenicol and mitomycin- c. Hydroxylamine has been used to induce pollen sterility and high yielding variants in crop plant[7-8]. Hydroxylamine has previously been used to increase the seed yield and other agronomic characters leading to the production of kiyanka, a commercial soviet variety of wheat. Previous studies[9], have reported that hydroquinone positively affects the maturity time and pod yield per plant.Many chemical mutagens have been employed for obtaining useful mutants in various crop species[10]. However the various workers emphasize that artificial induction of mutation by colchicines (Col), ethyl methane sulphonate (EMS), hydroquinone (HQ), hydroxylamine(HA) and sodium azide (SA) provides tool to overcome the limitations of variability in plants especially mutations that induce specific improvement without disturbing their preferred attributes[9, 11, 12].Soyabeans are one of the “biotech food” crops that have been genetically modified, and genetically modified soyabeans are being used in an increasing number of products. Soyabeans possesses good quality protein which is comparable to other protein foods and is suitable for all ages, infants to the elderly. Soyabean protein products also contain a high concentration of isoflavones, up to lg/kg[13]. The addition of soy protein in diet or replacing animal protein in the diet with soy lowers blood cholesterol and hence the risk of coronary diseases and cancer. Earlier works[14-16] reported an important link between soy consumption and a reduced risk of certain types of cancer. Asian women, who typically eat a soy based diet, have a much lower incidence of breast cancer than western women. Significant changes in the menstrual cycle of women who were fed on soy diet have also been reported[17]. The objective of this study is to enhance the genetic variability in soyabean (Glycine max) using hydroxylamine and hydroquinone to determine the optimal conditions for the induction of this genetic variability, in order to shorten the generation cycle of the crop and to increase yield through genetic modification.
2. Materials and Method
- Healthy and uniform seeds of soyabean were selected from a lot obtained from Ekpoma Main Market, Ekpoma, Esan West Local Government Area, Edo State, Nigeria. Sets of 30 seeds were exposed to concentrations of 0 (Control), 0.019 %, 0.038 %, 0.075 %, 0.150 %, 0.300 % of Hydroxylamine and Hydroquinone in Petri dishes for 24 hours with intermittent shaking. The treated seeds were washed with distilled water to remove excess chemical and toxic products and sown directly into potting bags up to the point of harvest. During the course of studies, the following parameters were carefully measured.
2.1. Germination and Survival Studies
- The study of germination was carried out from the second to forth day. The total number of emerged seeds in the different concentrations of the two chemicals (hydroxylamine and hydroquinone) was expressed as a mean of the control. Seeds showing the emergence of cotyledon from the soil surface were recorded as having emerged. Plants that survived the 21st day were recorded as having survival and the results of the treated plants was expressed as a percentage of the control
2.2. Agronomic Studies
- The parameters studied include: Plant Height, Number of days to flower Number of Leaves, Number of flowers per Plant, Number of pods per Plant
2.3. Biochemical Study
- Determination of Chlorophyll Content of LeavesChlorophyll determination took place at the onset of flower bud initiation. From each experiment, Leaves from five randomly selected plants were collected fresh very early in the morning (7.00 am) for each treatment, stored in ice packs in the field and transported to the laboratory for analysis. Chlorophyll determination was carried out following the method of Lichtenthaler and Buschmann[18]. Two leaf discs of 3mm radius obtained from the third youngest leaf of each plant from each of the treatments were ground, extracted with 5ml of 80% w/w acetone and 20% water and centrifuged at 2,500r.p.m for three minutes. The clear supernatant was collected and the absorbance was determined at 645nm and 663nm on a Bosch and lamb spectronic 20 spectrophotometer using 80% w/w acetone and 20% water as reference blank. Calculation of total chlorophyll in two leaf punches For each plant, the chlorophyll was extracted from two leaf punches in 5 ml of solvent. The total Chl a and b concentrations in the 2 leaf punch extract was calculated based on Lichtenthaler and Buschmann[18] . Chl a = 12.25 A665 - 2.79 A645 = μg/ml in extract Chl a =[12.25 A665 - 2.79 A645] x 5 = total μg in 2 leaf punches Chl b = 21.5 A645 - 5.1 A665 = μg/ml in extract Chl b =[21.5 A645 - 5.1 A665] x 5 = total μg in 2 leaf punches For each leaf punch, radius = 3 mm = 0.003 m and surface area = π r2 = 3.14 x 0.0032 = 0.0000282 m2 Combined surface area of 2 leaf punches: 0.0000565 m2 Therefore, the total amount of Chl a+b (in μg) obtained from 2 leaf punches was multiplied by 1/0.0000565 = 17699.1 to obtain the concentration of Chl a+b in μg m-2. The value obtained was then converted to mg m-2. The average concentration for each treatment was then recorded as the total chlorophyll.
3. Result and Discussion
- The effects of hydroxylamine and hydroquinone on the parameters studied are presented below.
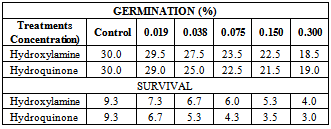
3.1. Germination and Survival Studies
- The germination studies showed that there were reductions or remarkable decrease in germination with increasing concentration of hydroxylamine and hydroquinone.
|
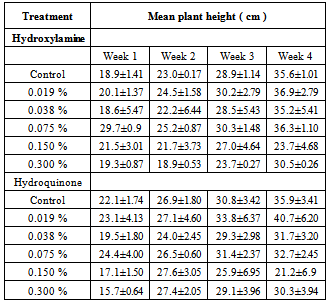
3.2. Plant Height
|
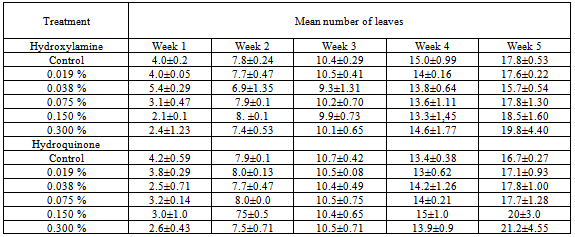
3.3. Number of Leaves / Plant
- The effect of hydroxylamine and hydroquinone on number of leaves is presented in Table 3. Increasing concentrations of the chemical mutagens (hydroxylamine and hydroquinone) led to gradual reduction in the number of leaves. Control treatments of both chemicals recorded the highest number of leaves. However, the chemical mutagens caused a slight increase in 0.3% in Week 5.
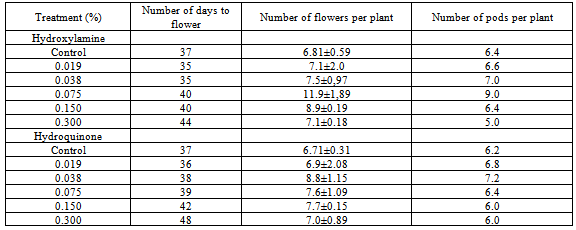
3.4. Number of Days to Flower
- The effects of hydroxylamine and hydroquinone on mean number of days to flower is presented in Table 4Increasing concentration of both chemical mutagens led to gradual reductions in the number of days to flower in 0.075 % by stimulating early initiation of flower bud. However, higher concentration of both chemical mutagens caused a delay in the number of days to flower in 0.150 % and 0.300 %. In the present study, early flowering variants have been recorded in both chemical mutagens under lower concentrations (0.019 % and 0.038 %). Early maturity mutants have previously been isolated under mutagenic treatment of some legume plants[24-25].
3.5. Number of Flowers Per Plant
- In the present investigation, 0.019% of both chemical mutagens recorded the highest number of flowers per plant when compared to the control as shown in table 4. Increase concentrations of the chemical mutagens led to decrease in the number of flower per plant.
|
|
|
3.6. Number of Pods Per Plant
- The effects of hydroxylamine and hydroquinone on mean number of pods per plant is presented in Table 4. Lower concentrations stimulated pod production whereas high concentrations reduced number of pods per plant compared to the control plants. The most effective concentration for hydroxylamine was 0.075 %. However for hydroquinone, the effective concentration which stimulates pod production was 0.038 %. The stimulatory effects of the chemicals were more pronounced in hydroxylamine than hydroquinone treated plants.
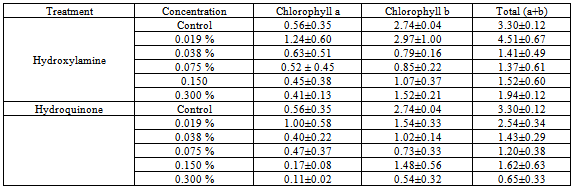
3.7. Chlorophyll Content
- The effects of hydroxylamine and hydroquinone on chlorophyll content are presented in Table 5. The general trend of both chemicals on chlorophyll content showed slight decreases with increasing concentration. However, 0.019% treated plants of hydroxyamine contain more chlorophyll content than the control plants.There was a decrease in the germination and survival of the plants as the concentrations increased. On the basis of the observation made on the effect of hydroxylamine and hydroquinone on soyabean (Glycine max (L.) Merr.), on the agronomic and yield parameters, it can be concluded that the two chemicals (hydroxylamine and hydroquinone) induced variability in the crop under study. The useful traits observed in the present work were early flowering under 0.019% and 0.038 % in both chemicals; both chemicals increased plant height under lower concentrations (0.019% and 0.075%), increase in the number of flowers per plant under 0.019% of both chemicals, increase in the number of pods per plant under 0.075% and 0.038% of hydroxylamine and hydroquinone respectively. Further studies would assist to have better understanding of inheritance pattern of these agronomic characters in soyabean so as to improve its genetic quality for the local farmers and hence improve on the present level of productivity of the crop.
4. Conclusions
- There was a decrease in the germination and survival of the plants as the concentrations of the two chemicals increased. On the basis of the observations made on the effects of hydroxylamine and hydroquinone on soyabean (Glycine max (L.) Merr.), on the agronomic and yield parameters, it can be concluded that the two chemicals (hydroxylamine and hydroquinone) induced variability in the crop under study. The useful traits observed in the present work were early flowering under 0.019 % and 0.038 % in both chemicals; both chemicals increased plant height under lower concentrations (0.019 % and 0.075 %), increase in the number of flowers per plant under 0.019 % of both chemicals, increase in the number of pods per plant under 0.075% and 0.038% of hydroxylamine and hydroquinone respectively. Further studies would assist to have better understanding of inheritance pattern of these agronomic and yield characters in soyabean so as to improve its genetic quality and hence the present level of productivity of the crop.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML