-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(5): 127-144
doi:10.5923/j.ijmb.20120205.02
Emerging Approaches to Inhibit Botrytis cinerea
Hana McFeeters, Robert L. McFeeters
Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL, 35899, USA
Correspondence to: Robert L. McFeeters, Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL, 35899, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Botrytis cinerea is a pathogenic fungus that has tremendous adverse impact on agriculture around the world. With susceptibility from seed to storage, highly adaptive nature under selective pressure, and ability to thrive at lower temperatures, B. cinerea is a formidable challenge. Technological advances in genetics, biochemistry, and biotechnology are providing new and unparalleled possibilities for treatment and management. With the resulting outburst of novel antifungal agents, it is difficult to keep abreast. This review not only catalogs and summarizes recent antifungal discoveries that target B. cinerea, but tries to give the reader a feel for how expansive the new possibilities are. Due to practical limitation, this review focuses on antifungal peptides/proteins and antifungal natural products. A brief perspective on emerging antifungals that hold potential to significantly impact the botryticide landscape is also included.
Keywords: Botrytis cinerea, Antifungal Peptides and Proteins, Natural Products, Emerging Antifungals
Cite this paper: Hana McFeeters, Robert L. McFeeters, Emerging Approaches to Inhibit Botrytis cinerea, International Journal of Modern Botany, Vol. 2 No. 5, 2012, pp. 127-144. doi: 10.5923/j.ijmb.20120205.02.
Article Outline
1. Introduction
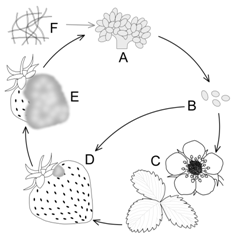
- Botrytis cinerea is a high risk necrotrophic pathogen known to damage over 230 plant species worldwide[1].This fungus can infect agriculturally important food crops including fruits, vegetables, cereals and even ornamental plants costing billions in damages yearly.The severity of outbreaks is dependent upon the prevailing environmental conditions.In severe epidemics, 100% yield loss can occur.B. cinerea can attack leaves, stems and fruit, often with heavy losses after harvest when crops are particularly vulnerable. Worse yet, it appears that B. cinerea enters a young host, remains quiescent, and then becomes active when the environment is conducive or plant physiology changes[1-3], Figure 1. B. cinerea is common in most climates manifesting itself as gray mold due to its property of producing a large number of spores.It sporulates profusely and dry conidia are dispersed through the air making this pathogen a constant threat to susceptible crops. Moreover, the ability of B. cinerea to be active at low temperatures (i.e. 0 ℃)[4] makes it a challenge for disease management during storage and shipment. Chemical control by several families of fungicides has been standard practice for many years.Applied doses vary from 400 to 3000 g/ha and the number of treatments during a season ranges from one or two, to more than twenty. Treatments of seeds or bulbs, as well as fungicide applications after harvest are common.The ability of B. cinerea to quickly adapt to new chemistries leads to the development of tolerant or resistant strains endangering long term fungicide use[5-11]. In general, drug resistance takes only a few years to develop[12]. Moreover, the application of fungicides has become increasingly unacceptable because of environmental, public health, and economicconsiderations making management of B. cinerea a serious challenge. Urgently needed alternative control measures are emerging, though in many instances the efficacy when applied alone has been inadequate and inconsistent.
2. Antifungal Peptides and Proteins
- Antifungal peptides and proteins are produced throughout the phylogenetic tree, from prokaryotes through higher eukaryotes.These peptides and proteins have a variety of amino acid sequences and multiple databases provide information about them[13-15].Historically, peptides and proteins have not been considered viable treatment candidates due to their costly manufacture.Advances in large scale recombinant expression (antifungal peptides and proteins made in bacterial or yeast systems and applied to crops in the field) and the growing transgenic possibilities (antifungal peptides and proteins expressed in modified crops) make peptide and protein biologics ever more feasible.As such, new research in the area has opened many new possibilities.Several reviews are available to acquaint the reader with certain subsets of antimicrobial peptides and proteins[16-20].This review focuses on antifungal peptides and proteins that have demonstrated activity against B. cinerea.
2.1. Single Celled Organisms: Yeast, Fungi, and Bacteria
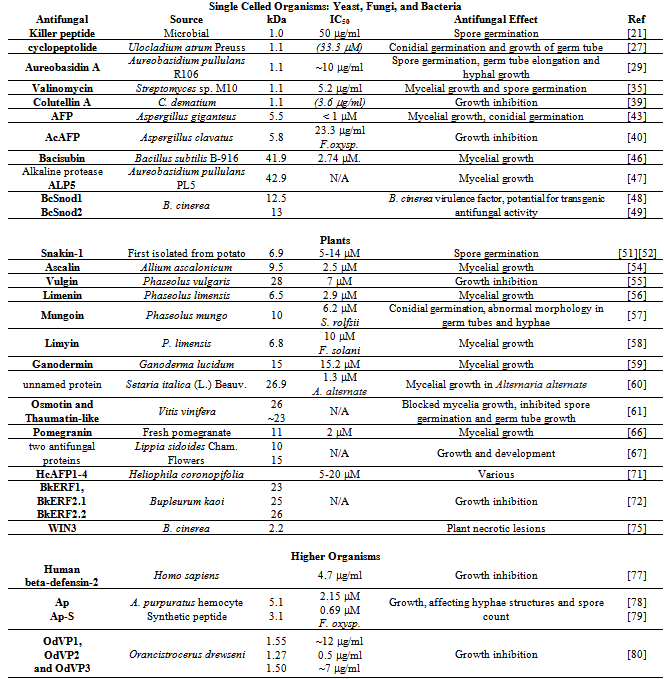
- Single celled organisms are abundant producers of antifungal agents.Part of their arsenal is a range of antifungal peptides and proteins.From the many antifungal peptides and proteins recently reported, those found active against B. cinerea are presented below.Derived from a single-chain antibody and acting as a yeast killer toxin, the 10 amino acid killer peptide was shown to exert activity against a broad spectrum of phytopathogenic fungi, including B. cinerea[21].Successful transgenic expression helped to demonstrate the killer peptide is a promising antifungal peptide.Expression in planta as a viral coat fusion protein using a potato virus system yielded chimeric virus particles displaying the peptide which exhibited enhanced antifungal activity.The authors further suggested that the potato virus system is an efficient method to produce and evaluate antimicrobial peptides in plants, an assertion that held true[22]and extended to human proteins.In fact, it appears that transient expression of foreign genes using plant viral vectors can produce immunogens impacting new vaccine development[23].For some time, the saprophytic fungus Ulocladium atrum Preuss has shown promise as a biological control agent for B. cinerea[24-26].More recently, the individual active antifungal components have been uncovered including a cyclopeptolide[27].This unnamed cyclopeptolide was most potent against B. cinerea with more moderate activity towards other fungi.It should be noted that this cyclopeptolide was originally identified as an antifungal substance against yeasts and yeast-like fungi[28].However, this report was the first to demonstrate its activity against filamentous fungi.Aureobasidin A is a cyclic depsipeptide produced by the yeast-like fungus Aureobasidium pullulansR106[29].It reduces conidial germination rates, delays conidial germination initiation, restricts germ tube and mycelium elongation, and induces abnormal morphology in germ tubes and hyphae.It has antifungal activity towards B. cinerea among other pathogenic fungi[30]and yeast[31].Though sensitivity was species dependent, 50 μg/ml was effective in reducing the incidence and severity of gray mold on strawberries after artificial inoculation[29].Aureobasidin A targets inositol phosphorylceramide synthase[32], an essential fungal enzyme absent from humans and other mammals.The gene encoding its biosynthesis complex has been characterized[33] and the high resolution structure solved[34]. Aided by an extensive background and supporting information, Aureobasidin A holds considerable promise as relatively safe crop antifungal. Valinomycin is also a depsipeptide antagonistic towards B. cinerea.It has been purified from culture extracts of Streptomyces sp. (M10), a gram-positive bacterial strain isolated from soil[35].It is a natural ionophore facilitating potassium ion movement through lipid membranes. Valinomycin showed in vitro antifungal activity against B. cinerea and also in vivo control efficacy against Botrytis blight development in cucumber plants.Disease control efficacy was similar to the commercial fungicide Vinclozolin. Long known to have antifungal activity[35], this was the first report on disease control against Botrytis blight.The genes responsible for biosynthesis are known[36] so transgenic implementation is possible.However, in planta expression may be limited by the supply of D-amino acids, though both enantiomers are found in plants[37, 38]. Colletotrichum dematium is an endophytic fungus from Costa Rica that makes Colutellin A[39].Colutellin A has a mass of 1.1 kDa and contains isoleucine, valine, serine, N-methyl-valine and beta-aminoisobutryic acid in a molar ratio of 3:2:1:1:1, respectively.It was isolated from culture broth by tracking antifungal activity.At 48 hours post exposure, naturally expressed Collutellin A has a MIC of 3.6 μg/ml against B. cinerea.The mold Aspergillus giganteus produces a basic low-molecular-weight peptide, generically designated AFP, that is structurally related to plant defensins and thionins[43].It has an approximate molecular weight of 5.5 kDa and is composed of 51 residues, 4 of which are cysteine. It is secreted as an inactive precursor containing an amino-terminal extension of 6 amino acids which is later removed to produce the active protein.AFP consists of 5 antiparallel beta strands forming a compact beta barrel that is stabilized by disulfide bonds[44] and has recently been recombinantly expressed in yeast[45].AFP inhibited B. cinerea with an IC50 below 1 μM at 24 hours[43].Mycelial growth and conidial germination were inhibited and application to plant leaves protected against Botrytis infection.An additive effect against the fungus was observed when AFP was combined with Cecropin A, a membrane disrupting peptide.These results indicate the potential of AFP and the afp gene to enhance protection against B. cinerea.Similar to AFP, a novel antifungal protein/peptide AcAFP is produced by the fungus Aspergillus clavatus[40].In the genome, the 282 base pair open reading is interrupted by two small introns.The cDNA coding for AcAFP represents a pre-pro-protein of 94 residues that is processed to a 51 amino acid, cysteine-rich mature product.AcAFP has molecular mass of 5.8 kDa and is very thermostable.Structural modeling[42] suggests a compact fold of five anti-parallel beta sheets forming a barrel stabilized by four internal disulfide bonds.A putative cation binding site and adjacent hydrophobic stretch may be responsible for dose-dependent cell wall alterations.AcAFP inhibits mycelial growth of B. cinerea, amongst other pathogenic fungi.Recombinant AcAFP production has been optimized and conditions affecting antifungal activity determined[41].Activity decreased at high ionic strength and in the presence of 10 mM of divalent cations (Mn2+, Fe2+ and Ca2+).Bacisubin is a 41.9 kDa antifungal protein isolated from cultures of gram-positive bacteria Bacillus subtilis strain B-916[46]. Bacisubin exhibited inhibitory activity on mycelial growth of B. cinerea (and multiple other fungi) with an IC50 value of 2.74 μM.Bacisubin function is unknown, though did demonstrate ribonuclease and hemagglutinating activities.An alkaline protease ALP5 from the yeast-like fungus Aureobasidium pullulans PL5 was shown to play an important role in biocontrol of B. cinerea[47].ALP5 is composed of 415 amino acids, has a calculated molecular weight of 42.9 kDa and an isoelectric point of 4.5. Recombinant expression yielded active, homogeneous ALP5 that inhibited mycelial growth of B. cinerea.Another pair of proteins with antifungal potential are the BcSnod1 and BcSnod2 virulence factors from B. cinerea[48, 49].These highly similar, ~13 kDa proteins are secreted by B. cinerea and have been shown to induce necrosis in plant hosts.The BcSnods are members of the Ceratoplatanin family of small phytotoxic proteins, acting quite the opposite of defensins.However, studies have shown that low level transgenic expression of BcSnod related proteins confer increased resistance to B. cinerea in plant hosts[50]. Therefore a new “desensitizing” approach may be possible for these antifungal proteins.
2.2. Plants
- Through their life cycle, plants employ multiple means to counter fungal infection.Diverse plants from around the globe have been found to produce antifungal peptides and proteins.A subset of this vast array of antifungal peptides and proteins is active against Gray Mold and described below. First isolated from potato over a decade ago[51], Snakin-1 is composed of 63 amino acids.It contains 12 cysteine residues, has a molecular weight of 6.9 kDa, and has some sequence motifs in common with kistrin and other hemotoxic snake venoms.Protein recombinantly expressed in bacteria exhibited antifungal activity against B. cinerea with an IC50 of 5-14 μM, similar to original reports of activity from native plant derived protein[51, 52].Snakin-1 was shown to be self-interacting, localizing in the plasma membrane and detailed analysis revealed effects on cell division, metabolism, and cell wall composition. Though overexpressing Snakin-1 in potato did not cause morphological differences, transgenic studies have not been promising[53].Ascalin was isolated from the bulbs of the shallot Allium ascalonicum[54].It has a molecular weight of 9.5 kDa with sequence similarity to chitinases from other Allium species. Ascalin demonstrated somewhat specific inhibition of mycelial growth in B. cinerea. with an IC50 of 2.5 μM. Vulgin is another antifungal protein purified from pinto beans[55].Like Ascalin, Vulgin bears some sequence homology to chitinases.At 28 kDa, Vulgin is considerably larger than Ascalin but similar in size to jack bean and peanut chitinases. Unlike Ascalin, Vulgin exerted antifungal activity toward multiple fungi including Mycosphaerella arachidicola, Fusarium oxysporum, and Coprinus comatus. Vulgin had the weakest inhibition against B. cinerea, with an IC50 of 7 μM.Limenin was isolated from shelf bean seeds[56].It has a molecular weight of 6.5 kDa and suppressed mycelial growth in B. cinerea, among other fungi, with an IC50 of 2.9 μM.Its mechanism of action is not known, though inhibited translation in a eukaryotic cell-free system.Also from a legume, Mungoin is a novel protease inhibitor isolated from mung bean (Phaseolus mungo) seeds[57].It has a molecular weight of 10 kDa and displayed inhibitory activity toward a variety of fungal species including B. cinerea.No follow-up publications were available for either of these antifungal proteins.From another legume, a 6.8 kDa peptide designated Limyin, with both antifungal and antiproliferative activities, was isolated from the large lima bean (P. limensis) legumes[58]. Limyin’s N-terminal amino acid sequence is highly homologous to plant defensin and defensin precursors.Limyin is thermostable up to 80 ℃ and suppresses mycelial growth.No IC50 was reported for B. cinerea.These three examples perpetuate the axiom that legumes produce an abundance of proteins and peptides with important biological activities, including a number of antifungal peptides and proteins[16]. Ganodermin, a 15 kDa antifungal protein, was isolated from the medical mushroom Ganoderma lucidum[59].The N-terminal sequence of Ganodermin somewhat resembles Lyophyllum antifungal protein and Eryngin with slight similarity to thaumatin and thaumatin-like proteins noted.It inhibited mycelial growth of B. cinerea, among several fungi, with an IC50 value of 15.2 μM.The mechanism of antifungal inhibition is not known, though common activities including hemagglutinating (lectin), deoxyribonuclease, ribonuclease or protease inhibition were not observed.An antifungal protein with a molecular mass of 26.9 kDa was isolated from dry seeds of the foxtail millet Setaria italica (L.) Beauv.[60].The unnamed protein inhibited mycelial growth and exhibited antifungal activity against B. cinerea among other fungi.Observations from light and atomic force microscopy indicated that the mode of antifungal activity centered on attack on the fungal cell wall and retraction of cytoplasm in the hyphae leading to death of the mycelium.The modification caused by the foxtail millet antifungal protein may be related to interference of cell wall synthesis, affecting fungal morphogenesis and growth.Osmotin and another Thaumatin-like protein were isolated from grapevine (Vitis vinifera)[61].Both exhibited antifungal activity against B. cinerea.Upon fungal infection in grapevine leaves, both are induced and accumulate in the leaves and berries.These relatively well established antifungal proteins blocked mycelial growth, inhibited spore germination and germ tube growth of B. cinerea.Thaumatin and Thaumatin-like proteins have been transgenically expressed, furthering their progress as biocontrol agents.A notable finding was that in combination, these antifungal proteins display synergism[61] possibly providing insight into the rather wide range of fungal processes thaumatin-like proteins are involved in[62].Osmotin is a homolog of mammalian adiponectin that induces apoptosis in yeast[63]. It exerts its biological activity through mammalian adiponectin receptor homologs that are part of a RAS2/ cAMP signaling pathway[63, 64], in part weakening defensive cell wall barriers[65].This adds another dimension to the use of Osmotin in that it can be combined with other cell wall damaging antifungal agents for potentially increased efficacy.Pomegranin, isolated from fresh pomegranate peels, is an 11 kDa protein that inhibits mycelial growth in B. cinerea with an IC50 of 2 μM[66].The N-terminal amino acid sequence resembles a rice disease resistance nucleotide binding leucine-rich repeat (NB-LRR)-like protein.Similarly, two novel antifungal proteins that showed sequence homology to NB-LRR proteins were isolated and characterized from rosemary pepper (Lippia sidoides Cham.) flowers[67].They were 10 and 15 kDa in size and were able to inhibit B. cinerea development.Many highly variable NB-LRR disease resistance proteins are encoded in plant genomes.Also, plant NB-LRR immune receptors recognize a range of effector proteins from different pathogens and are incredibly adaptive in pathogen recognition and defense initiation[68].A growing body of evidence suggests the N-termini of NB-LRR proteins also function in pathogen recognition[69].Directly applicable, Pomegranin may be important in defining pathogen recognition specificity.It also appears that two NB-LRRs can function together to mediate disease resistance and only fragments of NB-LRR proteins are sufficient to initiate defense signaling[70].It should be noted, however, that the NB-LRR fragment sufficient for function is distinct between different NB-LRRs.Following the multiple redundant theme, four defensins designated HcAFP1-4 have been isolated from the flowering plant Heliophila coronopifolia, a native South African Brassicaceae species[71].Overall the peptides were 72% similar.However HcAFP-1 and -3 shared 94% homology and were unique in the Brassicaceae defensins.HcAFP2 and 4 were similar with Arabidopsis and Raphanus defensins. Homology modeling showed that the variable amino acids between the four HcAFP peptides altered surface properties. Moreover, the variability is located in regions thought to be responsible for determining specific activities for defensins. Activity against B. cinerea included membrane permeabilization, hyper-branching, and biomass reduction. Related to the similarity grouping, HcAFP2 and 4 caused membrane permeabilization whereas HcAFP1 and 3 caused mild morphogenetic effects, without any indication of membrane activity.Moreover, expression is tissue-specific and similarly grouped with HcAFP1 and 3 expressed in leaves, stems and flowers, whereas HcAFP2 and 4 expressed in seedpods and seeds.This suggests protective roles in unknown developmental and physiological processes. Recombinant HcAFP2 and 4 peptides showed IC50 values of 5-20 μg/ml, whereas HcAFP1 and 3 were less potent.Three novel ethylene response factor genes, BkERF1, BkERF2.1 and BkERF2.2, were isolated from the medicinal plant Bupleurum kaoi[72].They are ubiquitously expressed at low levels in all parts of mature plants, with BkERF2.2 moderately expressed in vegetative tissues. BkERFs contain a nuclear localization signal, an ERF/AP2 DNA binding domain and function as transcriptional activators.Transgenic overexpression of BkERFs enhanced resistance to B. cinerea providing evidence that BkERFs mediate the expression of defense-related genes in plants. ERF proteins are thought to integrate signals from jasmonic acid, ethylene, and salicylic acid defense regulated pathways[73, 74].The salicylic acid regulatory gene HopW1-1-interacting3, or WIN3, controls broad-spectrum disease resistance to B. cinerea[75].Previously shown to confer resistance to the Pseudomonas syringae, WIN3 acts additively with several salicylic acid regulators in regulating salicylic acid accumulation, cell death, and/or disease resistance.In Arabidopsis, salicylic acid mediated signaling is required for local resistance to B. cinerea[76]. Surprisingly, WIN3 affects flowering time and thus represents a novel node in salicylic acid signaling network regulating defense and development. It makes sense that immunity and development are connected, regulation of both being important for plant fitness.
2.3. Other Higher Organisms
- Some higher order organisms also produce a wide array of other defense peptides and proteins.Those tested and showing activity against B. cinerea are described below.Human beta-defensin-2 is a small peptide with broad antimicrobial activity.Transgenic expression in A. thaliana was shown to impart increased resistance to B. cinereain vitro and the resistance was correlated to the level of human beta-defensin-2 produced[77].From known shared structural homology between human beta-defensins and plant defensins, functional homology was demonstrated between plant and mammalian defensins kingdoms for the first time.This opens the possibility of transgenic antifungals in plants to antifungals from other eukaryotic kingdoms.Such a cross species approach could be quite effective for furthering antifungal strategies.Ap is a 47 residue peptide of molecular weight 5.1 kDa isolated from the Chilean sea scallop Argopecten purpuratus hemocytes[78]. Based on the native sequence, a 30-residue synthetic peptide, Ap-S, was designed, that was much more active than native Ap.Ap-S has been expressed recombinantly and no cytotoxicity was observed in fish[79]. The recombinant protein inhibited B. cinerea growth at 81 nM by affecting hyphae structures and spore count.Three wasp venom peptides isolated from Orancistrocerus drewseni (Hymenoptera: Eumenidae) exhibited antifungal activities[80]. These peptides, designated OdVP1-3, contain a high content of positively charged and hydrophobic amino acids indicative of amphipathic alpha-helices. All three share typical characteristics of amidated C-termini proteins. Of note, amidization neutralizing negative charge on the C-terminus is a common posttranslational modification in peptide hormones and also found in multiple redundant neurotoxins isolated from snake venom[81].OdVP2 and a variant with two additional N-terminal amino acid residues showed the most potent antifungal activity, with IC50 values near 0.5 μg/ml.
2.4. Antifungal Peptide and Protein Summary Table
3. Antifungal Natural Products
- Plants possess a wide array of defense mechanisms and defensive countermeasures against pathogenic fungi.One category already discussed is the antifungal peptides and proteins.Another is the small molecule natural products. Generally termed secondary plant metabolites, this broadly defined category can be divided into numerous subsets based on source, chemistry, inhibition mechanism, etc.In this review, they are divided into biosurfactants, essential oils, volatile compounds, and other natural products.As with the antifungal peptides and proteins, many new small molecule natural products have been found to have antifungal activity in recent years.Some are modified versions of past successes and others represent new classes of molecules altogether. Those with reported activity against B. cinerea are included below.
3.1. Biosurfactants, Lipopeptides, and Lipid Related Antifungals
- Biosurfactants are amphipathic detergent-like molecules produced from natural sources.Popularity and interest in biosurfactants come from their widespread applicability, diversity, selectivity, low toxicity, environmentally friendly nature (biodegradability, biocompatibility), availability from large-scale production, and relative ease of preparation. Included in this section are the lipopeptides which are related to both biosurfactants and the antifungal peptides. Most are of natural origin though synthetic biosurfactants are also included.The rhamnolipids are simple glycolipids comprised of a fatty acid tail with one or two rhamnose rings at the carboxyl end of the fatty acid (see Figure 2A).Their antifungal activity comes from disruption of fungal cell membranes.Zoospores are especially vulnerable to rhamnolipids because they lack the protective cell wall present in other fungal life stages.Rhamnolipids produced by the gram-negative bacteria Pseudomonas aeruginosa triggered defense responses and protection against the fungus B. cinerea in grapevine[83] by inhibited spore germination and mycelium growth. Another Pseudomonas strain, Pseudomonas putida strain 267, also produces biosurfactants that lyse zoospores[84]. These biosurfactants, identified as cyclic lipopeptides resembling putisolvin, were also shown to inhibit B. cinerea growth.Isolated from fermented food, a gram-positive Bacillus subtilis strain was found to produce biosurfactants with antifungal activity[85].The biosurfactant activity was stable at high temperature and a wide range of pH and salt concentrations.In a subsequent report, this Bacillus subtilis strain was shown to inhibit mycelium growth of B. cinerea by 70%.Further analysis of active components lead to the characterization of a mixture lipopeptides and lipopeptide homologs[86].Another mixture of antifungal lipopeptides was isolated from a gram-negative Acinetobacter baumanniistrain[87].It was found that three antifungal lipopeptides were secreted, all isolated and identified as isomers of Iturin A (a pore-forming lipopeptide).Individual IC50 values were not reported though 50% growth inhibition of B. cinerea was observed at 25 μl of filtrate per milliliter of fungal culture. New antifungal agents have also been developed from microbial modification of polyunsaturated fatty acids. Cultures of Pseudomonas aeruginosa PR3 have been used to make docosahexaenoic acid, which inhibited mycelial growth and had a strong detrimental effect on spore germination in B. cinerea[90]. Minimum inhibitory concentrations are in the range of 125-500 μg/ml.
 | Figure 2. Structural diagram of (A) a rhamnolipid and (B) Neopeptins. Substituent 1 corresponds to Neopeptin A and substituent 2 to Neopeptin B |
3.2. Essential Oils and Volatile Compounds
- In addition to antifungal peptides/proteins and biosurfactants, essential oils and volatile compounds are another category of small molecule natural products with antifungal activity.Bioactivity in the vapor phase makes volatiles very attractive as possible postharvest fumigants. Interest is especially growing in the use of naturally derived essential oils[94] and volatile compounds as fungal control agents in agriculture, fueled by safety concerns regarding synthetic antifungals.To distinguish from volatile compounds, essential oils are the odorous and volatile products of plant secondary metabolism.Essential oils appear to have evolved to protect plants against invading pathogens[95].Widely used in traditional/folk medicine, their antifungal activity is well documented[96].Becoming somewhat dated, a past review covers antibacterial and antifungal activities of essential oils and their mechanisms of action[97]. As before, only more recently reported volatiles and only those that demonstrate activity against B. cinerea will be covered.Grouped in the categories of essential oils and volatile compounds, further subdivision is based on source.
3.2.1. Essential Oils
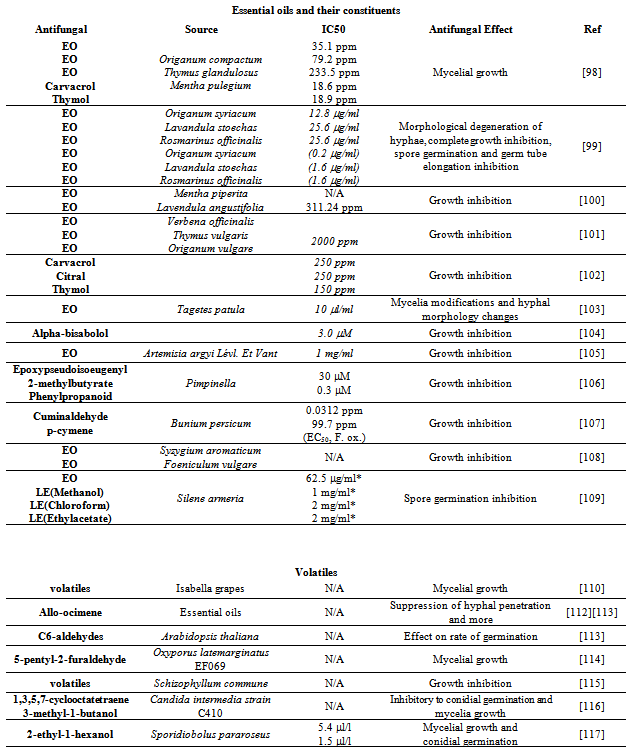
- From the fragrant nature of many herbs and spices, it is not surprising that they possess a variety of essential oils.It has long been known that certain spices and herbs have strong antiseptic properties.Moreover, extracts or compounds found in the extracts of numerous spices and herbs have been reported to show activity against B. cinerea.They are described below according to the plant origin.Lamiaceae, Verbenaceae: From Moroccan herbs of the Lamiaceae family, essential oils from Origanum compactum and Thymus glandulosus completely inhibited B. cinerea at 100 ppm with IC50 values of 35.1 and 79.2 ppm, respectively[98].The two main constituents, thymol and carvacrol, exhibited the strongest antifungal activity with complete inhibition for both at 100 ppm.These volatiles inhibited the growth of the mycelium.Essential oil of Mentha pulegium showed moderate inhibition with IC50 of 233.5 ppm.Essential oils obtained from other Lamiaceae family members (Origanum syriacum L. var. bevanii, Lavandula stoechas L. var. stoechas, and Rosmarinus officinalis L.) were also found to have activity against B. cinerea[99]. Different concentrations inhibited growth in a dose-dependent manner and the volatile phase was consistently more effective than the contact phase.Volatile vapor from Origanum oil completely inhibited B. cinerea growth at 0.2 μg/ml air whereas the essential oils of lavender and rosemary required concentrations of 1.6 μg/ml air.For the contact phase, the concentration of Origanum oil had to be 12.8 μg/ml to inhibit the growth of B. cinerea completely.Rosemary and lavender essential oils required roughly twice the concentration for complete inhibition in the contact phase.These essential oils cause morphological degeneration of hyphae including shriveling, cytoplasmic coagulation, vacuolations, and loss of conidiation.Spore germination and germ tube elongation were also inhibited.In vivo assays resulted in protection of tomato against B. cinerea.Essential oils from Mentha piperita (peppermint) and Lavendula angustifolia (lavender) also demonstrated antibotrytis activity[100].The strongest antifungal activity was from L. angustifolia, completely inhibiting B. cinerea at 1,000 ppm with an EC50 of 311.24 ppm.Similarly, essential oils from three Mediterranean aromatic plants (Verbena officinalis, Thymus vulgaris and Origanum vulgare) were found to harbor activity against phytopathogenic fungi[101]. In follow-up studies, the eight main components of these essential oils were tested against agents of post-harvest fruit decay including B. cinerea[102].Citral and carvacrol at 250 ppm and thymol at 150 ppm stopped B. cinerea growth.Asteraceae: Essential oils from several flowering plants have demonstrated antifungal activity against B. cinerea. Essential oil from the Indian marigold Tagetes patula, in the daisy family of Asteraceae, completely inhibited B. cinerea growth at 10 μl/ml[103].A multisite mechanism of action is proposed since ultrastructural modifications in mycelia and large alterations in hyphal morphology were observed.Also, the effects could not be attributed to any one of the two major components of the essential oil (piperitone and piperitenone). The effects could be a result of synergism between different chemical characteristics of the essential oil.In a related report, essential oils and two pure compounds (carvacrol and beta-bisabolol) from three different flowering Asteraceae demonstrated antifungal activity[104]. Subsequent evaluation indicated that alpha-bisabolol was weakly responsible for part of the B. cinerea growth inhibition.At a higher concentration, 1,000 mg/mL, essential oil of Artemisia argyi Lévl. et Vant inflorescence (wormwood) was also shown to inhibit B. cinerea growth[105].Apiaceae, Myrtaceae: Pimpinella is a genus in the Apiaceae family, commonly referred to as the carrot or parsley family.A new 'phenylpropanoid', 4-(3-methyl-oxiran-2-yl) phenyl 2-methylbutanoate was isolated from the essential oils of Turkish Pimpinella species[106].This compound together with epoxypseudoisoeugenyl 2-methyl-butyrate demonstrated growth inhibition against B. cinerea with IC50 values of approximately 0.3 μM and 30 μM at 48 hours for the phenylpropanoid and epoxy-butyrate, respectively. From a screen of fifty-two herbs and spices, volatiles from black zira exhibited the strongest inhibition of B. cinerea, followed by cumin and cardamom[107].Seven volatile compounds from black zira were detected with cuminaldehyde and p-cymene begin the most potent botryticides. Though EC50s were 99.7 ppm for p-cymene and 0.0312 ppm for cuminaldehyde against F. oxysporum, no values were reported for B. cinerea.Essential oils the Myrtaceae Syzygium aromaticum (cloves) and the Apiaceae Foeniculum vulgare (fennel) showed nearly identical antifungal activity against B. cinerea[108].The effects were dose dependent with 85% inhibition at 1 μL/mL after 10 days dropping to approximately 50% after 20 days for both. Caryophyllaceae: Antifungal activity was also reported for essential oil and organic extracts isolated from the floral parts of Silene armeria L. of the carnation family[109].B. cinerea growth inhibition ranged of 39.6-67.6%, along with their respective MIC values ranging from 62.5 to 1000 μg/ml. Strong detrimental effects on spore germination were observed.
3.2.2. Volatile Compounds
- Antifungal volatile compounds are very much related to essential oils, usually being derived from them.The major difference is that volatile compounds are individual compounds whereas essential oils are often uncharacterized mixtures.Volatiles from Isabella grapes were shown to reduce the inoculum and pathogenicity of B. cinerea[110,111]. Inhibitory action was on sporulation and sclerotia formation of the fungus, with pronounced inhibition at cooler temperatures. The active components were not identified and no follow-up has been published.Another antifungal volatile compound commonly found in essential oils is allo-ocimene. This compound was shown to enhance resistance of Arabidopsis thaliana against B. cinerea[112, 113].Hyphal penetration and growth were suppressed and allo-ocimene induced lignification and accumulation of antifungal substances, in particular camalexin.Moreover, allo-ocimene treatment resulted in more rapid and more intense responses to B. cinerea challenge, suggesting allo-ocimene primes defensive responses[113].The C6-aldehydes are another family of volatile compounds with antibotrytis activity[113].They are made by hydroperoxide lyase (HPL) and common to most terrestrial plants.Arabidopsis thaliana with upregulated HPL produced large quantities of these volatiles that were found to inhibit B. cinerea infection.This effect was proportional to the level of expression of HPL suggesting a direct fungicidal activity of C6-aldehydes.With the potential utility of volatile compounds as antifungals, the search for volatile compounds has expanded beyond plants. Other sources including bacteria and fungi have been reported to produce antifungal volatile compounds. 5-pentyl-2-furaldehyde was isolated from cultures of the fungus Oxyporus latemarginatus EF069[114]. Purified 5-pentyl-2-furaldehyde inhibited mycelial growth of B. cinerea in a dose-dependent manner. Mycofumigation with solid EF069 cultures reduced the development of postharvest B. cinerea in apples demonstrating the possible use of either Oxyporus latemarginatus EF069 or 5-pentyl-2-furaldehyde as a biocontrol agent.Another group of fungi, the Chilean saprobiontic fungi, were shown to have antifungal activity[115].Of those fungi tested, Schizophyllum commune emitted volatiles that exhibited the highest inhibitory activity against B. cinerea.Volatiles produced by the yeast Candida intermedia strain C410 suppressed B. cinerea growth[116].The two most abundant compounds, 1,3,5,7-cyclooctatetraene and 3-methyl-1-butanol, were highly inhibitory to conidial germination and mycelial growth. It was also shown that the incidence and severity of Botrytis fruit rot on strawberry was reduced by exposure of strawberry fruit to these volatiles, demonstrating biofumigant control for a volatile compound.The yeast Sporidiobolus pararoseus strain YCXT3 produces a volatile, 2-ethyl-1-hexanol, inhibiting mycelia growth and conidial germination of B. cinerea with IC50 values of 5.4 μl/l and 1.5 μl/l.The yeast cells were shown suppress B. cinerea infection of strawberry[117].
3.3. Other Natural Products
- In addition to essential oils and volatile compounds, several other small molecule natural products are deserving of mention.Tea polyphenols were reported to control Gray Mold in grape[118]. They significantly inhibited B. cinerea spore germination at higher concentrations and inhibited mycelium growth at lower concentrations. Glyceollins are a novel class of antiestrogenic phytoalexins from soybean. They had detrimental effects on B. cinerea spore germination, though no quantitation was reported[119]. Sixteen compounds isolated from the fermentation broth of the endophytic fungus Aspergillus fumigatus LN-4 showed antifungal activities[120]. Four of the compounds (12β-hydroxy-13α–methoxyver–ruculogenTR-2, fumitre-morgin B, verruculogen, and helvolic acid), exhibited antifungal activities with MIC values of 6.25-50 μg/mL.The structures of all compounds were determined and structure - activity relationships discussed in this report.
3.4. Essential Oil and Volatile Compound Summary Table
|
4. Emerging B. cinerea Antifungals
- Advances in biotechnology coupled with pathogen specific knowledge propel new antifungal approaches.What follows is a rapid fire summary of emerging possibilities that fall outside the previously discussed categories.The first section focuses on B. cinerea intracellular signaling, with better understanding of cellular pathways leading to increased potential for antifungal discovery.The second part centers on transgenic possibilities.The remainder deals with other prospects worthy of mention, but again not fitting in previous sections.
4.1. Insight into B. cinerea Intracellular Signaling with Potential for Intervention
- A good example for fungicide development based on understanding the intracellular signaling is the phenylpyrrole fungicide Fludioxonil.It activates the osmotic MAP kinase cascade via Hog1 (High osmolarity glycerol response)[121]. In Neurospora crassa, the Hog1 pathway regulates expression of a transcription factor downstream of the OS-2 MAP kinase involved in conidia stress tolerance[122].It is suggested to regulate genes involved in conidia formation, circadian rhythm, and ascospore maturation.A large number of genes dependent on the Hog1 MAPK cascade are modulated by Fludioxonil[123].In response to osmotic stress and Fludioxonil, expression of genes for glycerol synthesis (gcy-1, gcy-3, and dak-1), gluconeogenesis (fbp-1 and pck-1), and catalase (ctt-1) are activated[124].Fludioxonil also causes a plasma membrane hyperpolarization and H+ efflux, consistent with activation of the H+-ATPase. Concomitantly K+ uptake occurs and turgor (pressure that pushes the plasma membrane against the plant cell wall) increases about 2-fold above normal levels[125]. Better understanding of intracellular signaling has led to additive measures for antifungal treatment.Again in N. crassa, histidine kinase mutations conferred Fludioxonil resistance and osmotic sensitivity[126].Therefore a histidine kinase activating compound could be used to increase the longevity of Fludioxonil potency and delay onset of resistance development.In Cryptococcus neoformans, Hrk1 is analogous to Hog1.A hrk1Δ mutant exhibited almost complete resistance to Fludioxonil[127]. Interestingly, inhibition of Hrk1 substantially increasing azole drug susceptibility, providing another novel strategy for combining antifungals.Coapplication of 2,5-dihydroxybenzoic acid, an aspirin/salicylic acid metabolite, elevates the activity of Fludioxonil through disruption of cellular glutathione reduction/oxidization homeostasis[128]. Fludioxonil activity could also be enhanced by using berberine and phenolic compounds to target antioxidative stress response in fungi[129] or natural chemosensitizing agents targeting the oxidative stress-response system[130]. Redox-active compounds can serve as chemosensitizing agents to overcome resistance and lower effective fungicide dosages, reducing costs while lowering of environmental and health risks. Another emerging antibotrytis prospect centers on stress response processes.The ability to adapt to multiple stresses requires cross-talk and fine-tuning of stress signaling pathways. For pathogen signaling, several new interconnections have been uncovered. BcSak1 is a mitogen-activated protein kinase found in B. cinerea.The majority of genes regulated by BcSak1 are not involved in stress responses[131], however BcSak1 is involved in regulation of secondary metabolism with secretedphytotoxins reduced in deletion mutants.In planta experiments underlined the essential role of BcSak1 in the early stages of infection, showing it translocates to the nucleus and then changes to cytosolic distribution during hyphal growth within the tissue.Historically kinases are good drug targets, pointing to favorable possibilities for BcSak1 intervention.Another potential point for intervention is through Atf1-homologous basic region leucine zipper transcription factors. These act downstream of stress-activated mitogen-activated protein kinases and regulate transcription of general stress response genes.In B. cinerea, BcAtf1 is connected to the BcSak1, sharing stress response target genes[132]. Like BcSak1, BcAtf1 regulates a diversity of cellular processes including differentiation, though their roles are opposing.BcAtf1 knockout mutations also increased sensitivity to cell wall-interfering agents suggesting additional antifungal applications.Also involved in intracellular signaling are Bcpp2Ac (a catalytic subunit of a PP2A serine/threonine protein phosphatase) and SPT3 (a subunit of the Spt-Ada-Gcn5-Acetyl-transferase-like transcriptional regulator complex). Gene replacement and silencing approaches revealed that both are crucial for virulence, growth, and differentiation as well as for resistance to peroxide in B. cinerea[133].Their critical functions point to these genes as possible targets for antifungal development.
4.2. Transgenic
- Introducing another organism’s defense mechanisms into plants to increase protection against B. cinerea is a growing means of engineering resistance.Genetic modification does face societal reluctance in accepting modified crops. However, particularly promising is the transgenic expression of antifungal peptides or proteins since disruptions of normal metabolite flow are unlikely. Regardless, progress with transgenics continues to advance many types of disease resistance in plants, including antifungal development.Tobacco and tomato plants transformed with the Inhibitor of Virus Replication (IVR) gene become partially resistant to B. cinerea infection[134, 135]. IVR-like compounds were found in the certain species that were highly resistant to certain viruses.Plant protein extracts containing transgenically expressed SG2 chitinase from Bacillus pumilus displayed a high inhibitory effect on spore germination and hyphal growth in B. cinerea[136]. Antifungal activity of truncated SG2, lacking the chitin binding and fibronectin type III domains, was lost.Both forms showed essentially equal hydrolytic activity toward colloidal chitin.These findings demonstrate that chitin binding and fibronectin type III domains play an important role in hydrolysis of chitin-glucan complex of fungal cell walls.From Gerbera hybrida (Asteraceae), a newly cloned chalcone synthase-like polyketide synthase, 2-pyrone synthase was shown to be critical for inhibiting fungal infection[137].2-pyrone synthase is able to synthesize 4-hydroxy-6-methyl-2-pyrone (triacetolactone), a putative precursor for two abundant glucosides in gerbera.Gerbera plants lacking this gene are susceptible to B. cinerea infection and fail to produce several compounds induced upon infection with the mold.This synthase along with other members of the antifungal pathway could be used to generate fungal resistance with little toxicity expected from the new compounds introduced to the system for the Asteraceae plant family or others with similar pathways for phytoalexin generation.Arabidopsis possesses two arginase-encoding genes, ARGAH1 and ARGAH2. Arginases catalyze breakdown of arginine into ornithine and urea. Arabidopsis plants overexpressing arginase were less susceptible to B. cinerea and accumulation of arginase-encoding gene mRNA was observed in Arabidopsis upon inoculation with B. cinerea[138].These results provide new insights into amino acid metabolic changes under stress and another potential mechanism to disrupt B. cinerea.Believed to be involved in plant defense responses, lipid transfer proteins (LTPs) are members of the pathogenesis-related proteins (PR-14) family.A novel gene Ltp 3F1 encoding an antifungal protein from wheat (Sumai 3) was recombinantly expressed in bacteria[139].The LTP fusion protein exhibited a broad- spectrum antifungal activity including activity against B. cinerea.Transgenic expression resulted in tobacco plants with normal phenotype and detached leaves showing increased fungal resistance.The demonstrated transgenic effects and potential for broad-spectrum antifungal activity make this LTP a viable candidate for large scale use in crop plants.Another tool to advance transgenic antifungals are wound-inducible promoters, especially useful to limit production/accumulation of antimicrobial peptides and proteins to infected areas.A wound-inducible promoter was reported for transgenic expression of a new ribosome- inactivating protein[140].Direct antifungal toxicity and reduced B. cinerea leaf damage was observed.
4.3. Miscellaneous
- Not directly fitting into any previously described category, there are still several antifungal developments that are worth mentioning.They are listed chronologically here.A new discovery has added to understanding of salicylic acid and its role in resistance.The enhanced pseudomonas susceptibility gene, EPS1, was isolated and found to encode for a BAHD acyltransferase[141].Mutations of EPS1, similar for other genes important for salicylic acid accumulation or signaling, impart enhanced resistance to B. cinerea.The authors suggest there is natural variation among the host (Arabidopsis ecotypes) with respect to the antagonistic cross-talk between defense signaling pathways against various types of microbial pathogens.Further characterization of factors involved in the defense pathways will be needed to explain the molecular basis for the natural variation among different species.Computational chemistry studies have shown a clear structure-activity relationship between twenty-two chlorophenyl derivatives and B. cinerea antifungal activity[142]. Maximum fungal growth inhibition was exhibited for R and S enantiomers of 1-(4′-chloro-phenyl)-2-phenylethanol. Of larger perspective, a multiobjective optimization of the global antifungal profiles established a filtering strategy for new antifungal candidates.Nanosized silica hybrid silver complex (NSS), nanosilver bound to silica molecules, also showed strong antifungal activity[143].The growth of Rhizoctonia solani was decreased by more than 90% at 6 μg/ml of NSS added directly to the growth media.The antifungal effects were shown against B. cinerea, from which the antifungal mechanism of the complex was also determined.NSS solutions maintained stable antifungal activity for at least two years.Jasmonic acid is a defensin that in recent years has become known for its ability to impart disease resistance.Plants grown from seeds treated with jasmonic acid showed increased resistance against B. cinerea[144].Priming responses were long-lasting, with significant increases in resistance sustained in plants grown from treated seed for at least 8 weeks, and were associated with enhanced defense gene expression during pathogen attack. Long-term defense priming by seed treatments was not accompanied by reductions in growth, and may therefore be suitable for commercial applications.Several new targets have also emerged for botryticide intervention.The Arabidopsis histidine kinase 5, known to mediate stomatal responses to exogenous and endogenous signals in Arabidopsis thaliana, contributes to B. cinerea resistance[145].Therefore upregulation in Arabidopsis or transgenic expression in another host may provide a means of increasing resistance to B. cinerea.Similarly, secreted papain-like cysteine proteases are important in plant immunity. One such protease, RD21, was identified in tomato as critical for susceptibility to B. cinereainfection[146].Decreased expression or elimination of this gene product again could provide a means of limiting B. cinerea infectivity if altering RD21 does not disrupt normal cellular functions in tomato.
5. Conclusions
- With fungicide-resistant strains, demand to reduce pesticide use, and appearance of iatrogenic diseases, there is clear need to develop new and alternative methods for managing B. cinerea as well as other pathogenic fungi.From the growing wealth of information, such approaches are being realized for every step of agricultural production. Priming of seeds, biocontrol and transgenic expression of any number of resistance enhancing genes during growth, and post-harvest examples have all been provided.To gauge the widespread development of antifungals, one only need look at the journals referenced in this review.Even with these advances, control of B. cinerea remains extremely difficult.In many cases, rapid adaptation to antifungal compounds occurs.Moreover, the broad habitat range, different infection strategies that vary along with conditions,ability to attack crops at almost any stage of growth, and ability to affect all parts of a plant contribute to the tremendous flexibility of this pathogenic fungus.The large toolbox of enzymes and metabolites that B. cinerea exploits to overcome host defenses adds to the difficulty.As witnessed for antifungal agents reported here, even rather ineffective agents are potentially vastly more potent and efficacious when used in combination.In fact, increased reliability and decreased variability in treatment outcomes have already been shown for combinations of control agents[147]. Only with continued advances including knowledge of the biology and epidemiology of B. cinerea along with new antifungal development will the promise to safeguard agricultural productivity for generations to come be met.
ACKNOWLEDGEMENTS
- The authors thank Dr. Maria Davis for introduction to the world of Botrytis cinerea.Her memory shines bright.
References
| [1] | Y. Elad, "Responses of plants to infection by Botrytis cinerea and novel means involved in reducing their susceptibility to infection.", Biological Reviews, vol. 72, no. pp. 381-422, 1997 |
| [2] | H. Forster and J. E. Adaskaveg, "Early brown rot infections in sweet cherry fruit are detected by monilinia-specific DNA primers", Phytopathology, vol. 90, no. 2, pp. 171-8, 2000 |
| [3] | B. Williamson, "Latency and quiescence in survival and success of fungal plant pathogens", CAB International, Oxford, UK, vol. no. pp. 187-207, 1994 |
| [4] | C. Brooks, Cooley J. S., "Temperature relations of apple-rot fungi", Journal of Agricultural Research, vol. 8, no. pp. 139-164, 1917 |
| [5] | H.-J. Rosslenbroich and D. Stuebler, "Botrytis cinerea * history of chemical control and novel fungicides for its management", Crop Protection, vol. 19, pp. 557-561, 2000 |
| [6] | P. Leruox, F. Chapeland, F. Arnold and M. Gredt, "ReHsistance de Botrytis cinerea aux fongicides. Du laboratoire au vignoble et vice versa.", Phytoma, vol. 504, pp. 62-67, 1998 |
| [7] | J. Rueegg, H.P. Lauber, W. Siegfried, O. Viret and U. Hilber, "Experiences with anilinopyrimidines in Switzerland", Pesticide Outlook, vol. 8, no. 3, pp. 28-33, 1997 |
| [8] | B. Forster and T. Staub, "Basis for use strategies of anilinopyrimidine and phenylpyrrole fungicides against Botrytis cinerea.", Crop Protection, vol. 15, no. 6, pp. 529- 537, 1996 |
| [9] | U. W. Hilber and M. Hilber-Bodmer, "Genetic basis and monitoring of resistance of Botryotinia fuckeliana to anilinopyrimidines", Plant Disease, vol. 82, no. 5, pp. 496-500, 1998 |
| [10] | P. Leroux and M. Gredt, "Etude in vitro de la resistance de Botrytis cinerea aux fongicides anilinopyrimidines", Agronomie, vol. 15, pp. 367-370, 1995 |
| [11] | A. Suty, R. Pontzen and K. Stenzel, "KBR2738: Mode d'action et sensibilite de Botrytis cinerea", Tours, France, pp. 1997 |
| [12] | P. Leroux, "Chemical Control fo Botrytis and its Resistance to Chemical Fungicides", Springer, Dordrecht, The Netherlands, 2007 |
| [13] | C. D. Fjell, R. E. Hancock and A. Cherkasov, "AMPer: a database and an automated discovery tool for antimicrobial peptides", Bioinformatics, vol. 23, no. 9, pp. 1148-55, 2007 |
| [14] | Z. Wang and G. Wang, "APD: the Antimicrobial Peptide Database", Nucleic Acids Research, vol. 32, Database issue, pp. D590-2, 2004 |
| [15] | M. Brahmachary, S. P. Krishnan, J. L. Koh, A. M. Khan, S. H. Seah, T. W. Tan, V. Brusic and V. B. Bajic, "ANTIMIC: a database of antimicrobial sequences", Nucleic Acids Research, vol. 32, no. Database Issue, pp. D586-9, 2004 |
| [16] | T. B. Ng, "Antifungal proteins and peptides of leguminous and non-leguminous origins", Peptides, vol. 25, no. 7, pp. 1215-1222, 2004 |
| [17] | J. H. Wong, T. B. Ng, R. C. Cheung, X. J. Ye, H. X. Wang, S. K. Lam, P. Lin, Y. S. Chan, E. F. Fang, P. H. Ngai, L. X. Xia, X. Y. Ye, Y. Jiang and F. Liu, "Proteins with antifungal properties and other medicinal applications from plants and mushrooms", Applied Microbiology and Biotechnology, vol. 87, no. 4, pp. 1221-35, 2010 |
| [18] | C. P. Selitrennikoff, "Antifungal proteins", Applied and Environmental Microbiology, vol. 67, no. 7, pp. 2883-94, 2001 |
| [19] | A. J. De Lucca, T. E. Cleveland and D. E. Wedge, "Plant-derived antifungal proteins and peptides", Canadian Journal of Microbiology, vol. 51, no. 12, pp. 1001-14, 2005 |
| [20] | F. Marx, "Small, basic antifungal proteins secreted from filamentous ascomycetes: a comparative study regarding expression, structure, function and potential application", Applied Microbiology and Biotechnology, vol. 65, no. 2, pp. 133-42, 2004 |
| [21] | Marcello Donini, Chiara Lico, Selene Baschieri, Stefania Conti, Walter Magliani, Luciano Polonelli and Eugenio Benvenuto, "Production of an Engineered Killer Peptide in Nicotiana benthamiana by Using a Potato virus X Expression System", Applied and Environmental Microbiology, vol. 71, no. 10, pp. 6360-6367, 2005 |
| [22] | H. P. van Esse, "Identification of HR-inducing cDNAs from plant pathogens via a Gateway(®)-compatible binary Potato virus X-expression vector", Methods in Molecular Biology, vol. 835, pp. 97-105, 2012 |
| [23] | N. Cerovska, H. Hoffmeisterova, T. Moravec, H. Plchova, J. Folwarczna, H. Synkova, H. Ryslava, V. Ludvikova and M. Smahel, "Transient expression of Human papillomavirus type 16 L2 epitope fused to N- and C-terminus of coat protein of Potato virus X in plants", Journal of Biosciences, vol. 37, no. 1, pp. 125-33, 2012 |
| [24] | J. Kohl, M. Gerlagh, B. H. De Haas and M. C. Krijger, "Biological Control of Botrytis cinerea in Cyclamen with Ulocladium atrum and Gliocladium roseum Under Commercial Growing Conditions", Phytopathology, vol. 88, no. 6, pp. 568-75, 1998 |
| [25] | P. Berto, M. H. Jijakli and P. Lepoivre, "Possible Role of Colonization and Cell Wall-Degrading Enzymes in the Differential Ability of Three Ulocladium atrum Strains to Control Botrytis cinerea on Necrotic Strawberry Leaves", Phytopathology, vol. 91, no. 11, pp. 1030-6, 2001 |
| [26] | C. Metz, E. C. Oerke and H. W. Dehne, "Biological control of grey mould (Botrytis cinerea) with the antagonist Ulocladium atrum", Mededelingen, vol. 67, no. 2, pp. 353-9, 2002 |
| [27] | B. S. Yun, E. M. Kwon, J. C. Kim and S. H. Yu, "Antifungal cyclopeptolide from fungal saprophytic antagonist Ulocladium atrum", Journal of Microbiology and Biotechnology, vol. 17, no. 7, pp. 1217-20, 2007 |
| [28] | G. Emmer, M. A. Grassberger, J. G. Meingassner, G. Schulz and M. Schaude, "Derivatives of a novel cyclopeptolide. 1. Synthesis, antifungal activity, and structure-activity relationships", Journal of Medicinal Chemistry, vol. 37, no. 13, pp. 1908-17, 1994 |
| [29] | X. Liu, J. Wang, P. Gou, C. Mao, Z. R. Zhu and H. Li, "In vitro inhibition of postharvest pathogens of fruit and control of gray mold of strawberry and green mold of citrus by aureobasidin A", International Journal of Food Microbiology, vol. 119, no. 3, pp. 223-9, 2007 |
| [30] | K. Takesako, K. Ikai, F. Haruna, M. Endo, K. Shimanaka, E. Sono, T. Nakamura, I. Kato and H. Yamaguchi, "Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties", The Journal of Antibiotics, vol. 44, no. 9, pp. 919-24, 1991 |
| [31] | M. Endo, K. Takesako, I. Kato and H. Yamaguchi, "Fungicidal action of aureobasidin A, a cyclic depsipeptide antifungal antibiotic, against Saccharomyces cerevisiae", Antimicrobial Agents Chemotherapy, vol. 41, no. 3, pp. 672-6, 1997 |
| [32] | P. A. Aeed, C. L. Young, M. M. Nagiec and A. P. Elhammer, "Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A", Antimicrobial Agents Chemotherapy, vol. 53, no. 2, pp. 496-504, 2009 |
| [33] | J. L. Slightom, B. P. Metzger, H. T. Luu and A. P. Elhammer, "Cloning and molecular characterization of the gene encoding the Aureobasidin A biosynthesis complex in Aureobasidium pullulans BP-1938", Gene, vol. 431, no. 1-2, pp. 67-79, 2009 |
| [34] | K. Ikai, K. Takesako, K. Shiomi, M. Moriguchi, Y. Umeda, J. Yamamoto, I. Kato and H. Naganawa, "Structure of aureobasidin A", The Journal of Antibiotics, vol. 44, no. 9, pp. 925-33, 1991 |
| [35] | R. Brown, J. Brennan and C. Kelley, "An antifungal agent identical with valinomycin", Antibiotics and Chemotherapy, vol. 12, pp. 482-7, 1962 |
| [36] | J. B. Perkins, S. K. Guterman, C. L. Howitt, V. E. Williams, 2nd and J. Pero, "Streptomyces genes involved in biosynthesis of the peptide antibiotic valinomycin", Journal of Bacteriology, vol. 172, no. 6, pp. 3108-16, 1990 |
| [37] | H. Bruckner and T. Westhauser, "Chromatographic determination of L- and D-amino acids in plants", Amino Acids, vol. 24, no. 1-2, pp. 43-55, 2003 |
| [38] | T. Robinson, "D-amino acids in higher plants", Life Sciences, vol. 19, no. 8, pp. 1097-1102, 1976 |
| [39] | Y. Ren, G. A. Strobel, J. C. Graff, M. Jutila, S. G. Park, S. Gosh, D. Teplow, M. Condron, E. Pang, W. M. Hess and E. Moore, "Colutellin A, an immunosuppressive peptide from Colletotrichum dematium", Microbiology, vol. 154, no. Pt 7, pp. 1973-9, 2008 |
| [40] | H. Skouri-Gargouri and A. Gargouri, "First isolation of a novel thermostable antifungal peptide secreted by Aspergillus clavatus", Peptides, vol. 29, no. 11, pp. 1871-7, 2008 |
| [41] | H. Skouri-Gargouri, N. Jellouli-Chaker and A. Gargouri, "Factors affecting production and stability of the AcAFP antifungal peptide secreted by Aspergillus clavatus", Applied Microbiology and Biotechnology, vol. 86, no. 2, pp. 535-43, 2010 |
| [42] | H. Skouri-Gargouri, M. Ben Ali and A. Gargouri, "Molecular cloning, structural analysis and modelling of the AcAFP antifungal peptide from Aspergillus clavatus", Peptides, vol. 30, no. 10, pp. 1798-804, 2009 |
| [43] | A. B. Moreno, A. M. Del Pozo, M. Borja and B. S. Segundo, "Activity of the Antifungal Protein from Aspergillus giganteus Against Botrytis cinerea", Phytopathology, vol. 93, no. 11, pp. 1344-53, 2003 |
| [44] | R. Campos-Olivas, M. Bruix, J. Santoro, J. Lacadena, A. Martinez del Pozo, J. G. Gavilanes and M. Rico, "NMR solution structure of the antifungal protein from Aspergillus giganteus: evidence for cysteine pairing isomerism", Biochemistry, vol. 34, no. 9, pp. 3009-21, 1995 |
| [45] | B. Lopez-Garcia, A. B. Moreno, B. San Segundo, V. De los Rios, J. M. Manning, J. G. Gavilanes and A. Martinez-del-Pozo, "Production of the biotechnologically relevant AFP from Aspergillus giganteus in the yeast Pichia pastoris", Protein Expression and Purification, vol. 70, no. 2, pp. 206-10, 2010 |
| [46] | Y. Liu, Z. Chen, T. B. Ng, J. Zhang, M. Zhou, F. Song and F. Lu, "Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916", Peptides, vol. 28, no. 3, pp. 553-9, 2007 |
| [47] | D. Zhang, D. Spadaro, S. Valente, A. Garibaldi and M. L. Gullino, "Cloning, characterization, expression and antifungal activity of an alkaline serine protease of Aureobasidium pullulans PL5 involved in the biological control of postharvest pathogens", International Journal of Food Microbiology, vol. 153, no. 3, pp. 453-64, 2012 |
| [48] | M. Frias, C. Gonzalez and N. Brito, "BcSpl1, a cerato-platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host", The New Phytologist, vol. 192, no. 2, pp. 483-95, 2011 |
| [49] | S. Rathi, H. McFeeters, R. L. McFeeters and M. R. Davis, "Purification and Phytotoxic Analysis of Botrytis cinerea Virulence Factors: New Avenues for Crop Protection", Agriculture, vol. 2, no. 3, pp. 154-164, 2012 |
| [50] | J. S. Jeong, T. K. Mitchell and R. A. Dean, "The Magnaporthe grisea snodprot1 homolog, MSP1, is required for virulence", FEMS Microbiology Letters, vol. 273, no. 2, pp. 157-65, 2007 |
| [51] | Ana Segura, Manuel Moreno, Francisco Madueno, Antonio Molina and Francisco Garcia-Olmedo, "Snakin-1, a Peptide from Potato That Is Active Against Plant Pathogens", Molecular Plant-Microbe Interactions, vol. 12, no. 1, pp. 16-23, 1999 |
| [52] | N. Kovalskaya and R. W. Hammond, "Expression and functional characterization of the plant antimicrobial snakin-1 and defensin recombinant proteins", Protein Expression and Purification, vol. 63, no. 1, pp. 12-7, 2009 |
| [53] | V. Nahirnak, N. I. Almasia, P. V. Fernandez, H. E. Hopp, J. M. Estevez, F. Carrari and C. Vazquez-Rovere, "Potato snakin-1 gene silencing affects cell division, primary metabolism, and cell wall composition", Plant Physiology, vol. 158, no. 1, pp. 252-63, 2012 |
| [54] | H. X. Wang and T. B. Ng, "Ascalin, a new anti-fungal peptide with human immunodeficiency virus type 1 reverse transcriptase-inhibiting activity from shallot bulbs", Peptides, vol. 23, no. 6, pp. 1025-9, 2002 |
| [55] | X. Y. Ye and T. B. Ng, "Isolation of vulgin, a new antifungal polypeptide with mitogenic activity from the pinto bean", Journal of Peptide Science, vol. 9, no. 2, pp. 114-9, 2003 |
| [56] | J. H. Wong and T. B. Ng, "Limenin, a defensin-like peptide with multiple exploitable activities from shelf beans", Journal of Peptide Science, vol. 12, no. 5, pp. 341-6, 2006 |
| [57] | S. Wang, J. Lin, M. Ye, T. B. Ng, P. Rao and X. Ye, "Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities", Peptides, vol. 27, no. 12, pp. 3129-36, 2006 |
| [58] | Shaoyun Wang, Pingfan Rao and Xiuyun Ye, "Isolation and biochemical characterization of a novel leguminous defense peptide with antifungal and antiproliferative potency", Applied Microbiology and Biotechnology, vol. 82, no. 1, pp. 79-86, 2009 |
| [59] | H. Wang and T. B. Ng, "Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum", Peptides, vol. 27, no. 1, pp. 27-30, 2006 |
| [60] | Wentao Xu, Lu Wei, Wei Qu, Zhihong Liang, Jinai Wang, Xiaoli Peng, Yanan Zhang and Kunlun Huang, "A novel antifungal peptide from foxtail millet seeds", Journal of the Science of Food and Agriculture, vol. 91, no. 9, pp. 1630-1637, 2011 |
| [61] | S. Monteiro, M. Barakat, M. A. Picarra-Pereira, A. R. Teixeira and R. B. Ferreira, "Osmotin and thaumatin from grape: A putative general defense mechanism against pathogenic fungi", Phytopathology, vol. 93, no. 12, pp. 1505-1512, 2003 |
| [62] | J. J. Liu, R. Sturrock and A. K. Ekramoddoullah, "The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function", Plant Cell Reports, vol. 29, no. 5, pp. 419-36, 2010 |
| [63] | M. L. Narasimhan, M. A. Coca, J. Jin, T. Yamauchi, Y. Ito, T. Kadowaki, K. K. Kim, J. M. Pardo, B. Damsz, P. M. Hasegawa, D. J. Yun and R. A. Bressan, "Osmotin is a homolog of mammalian adiponectin and controls apoptosis in yeast through a homolog of mammalian adiponectin receptor", Molecular Cell, vol. 17, no. 2, pp. 171-80, 2005 |
| [64] | M. Miele, S. Costantini and G. Colonna, "Structural and functional similarities between osmotin from Nicotiana tabacum seeds and human adiponectin", PLoS ONE, vol. 6, no. 2, pp. e16690, 2011 |
| [65] | D. J. Yun, J. I. Ibeas, H. Lee, M. A. Coca, M. L. Narasimhan, Y. Uesono, P. M. Hasegawa, J. M. Pardo and R. A. Bressan, "Osmotin, a plant antifungal protein, subverts signal transduction to enhance fungal cell susceptibility", Molecular Cell, vol. 1, no. 6, pp. 807-17, 1998 |
| [66] | G. Guo, H. X. Wang and T. B. Ng, "Pomegranin, an antifungal peptide from pomegranate peels", Protein and Peptide Letters, vol. 16, no. 1, pp. 82-5, 2009 |
| [67] | J. S. Moreira, R. G. Almeida, L. S. Tavares, M. O. Santos, L. F. Viccini, I. M. Vasconcelos, J. T. Oliveira, N. R. Raposo, S. C. Dias and O. L. Franco, "Identification of botryticidal proteins with similarity to NBS-LRR proteins in rosemary pepper (Lippia sidoides Cham.) flowers", Protein Journal, vol. 30, no. 1, pp. 32-8, 2011 |
| [68] | J. Caplan, M. Padmanabhan and S. P. Dinesh-Kumar, "Plant NB-LRR immune receptors: from recognition to transcriptional reprogramming", Cell Host & Microbe, vol. 3, no. 3, pp. 126-35, 2008 |
| [69] | S. M. Collier and P. Moffett, "NB-LRRs work a 'bait and switch' on pathogens", Trends in Plant Science, vol. 14, no. 10, pp. 521-9, 2009 |
| [70] | T. K. Eitas and J. L. Dangl, "NB-LRR proteins: pairs, pieces, perception, partners, and pathways", Current Opinion in Plant Biology, vol. 13, no. 4, pp. 472-7, 2010 |
| [71] | A. de Beer and M. A. Vivier, "Four plant defensins from an indigenous South African Brassicaceae species display divergent activities against two test pathogens despite high sequence similarity in the encoding genes", BMC Research Notes, vol. 4, no. 1, pp. 459, 2011 |
| [72] | Wen-Yu Liu, Shu-Jiau Chiou, Chia-Yun Ko and Tsai-Yun Lin, "Functional characterization of three ethylene response factor genes from Bupleurum kaoi indicates that BkERFs mediate resistance to Botrytis cinerea", Journal of Plant Physiology, vol. 168, no. 4, pp. 375-381, 2011 |
| [73] | O. Lorenzo, R. Piqueras, J. J. Sanchez-Serrano and R. Solano, "Ethylene Response Factor1 integrates signals from ethylene and jasmonate pathways in plant defense", The Plant Cell, vol. 15, no. 1, pp. 165-78, 2003 |
| [74] | K. C. McGrath, B. Dombrecht, J. M. Manners, P. M. Schenk, C. I. Edgar, D. J. Maclean, W. R. Scheible, M. K. Udvardi and K. Kazan, "Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression", Plant Physiology, vol. 139, no. 2, pp. 949-59, 2005 |
| [75] | G. F. Wang, S. Seabolt, S. Hamdoun, G. Ng, J. Park and H. Lu, "Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering time in Arabidopsis", Plant Physiology, vol. 156, no. 3, pp. 1508-19, 2011 |
| [76] | S. Ferrari, J. M. Plotnikova, G. De Lorenzo and F. M. Ausubel, "Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4", The Plant Journal, vol. 35, no. 2, pp. 193-205, 2003 |
| [77] | An Aerts, Karin Thevissen, Sara Bresseleers, Jan Sels, Piet Wouters, Bruno Cammue and Isabelle François, "Arabidopsis thaliana plants expressing human beta- defensin-2 are more resistant to fungal attack: functional homology between plant and human defensins", Plant Cell Reports, vol. 26, no. 8, pp. 1391-1398, 2007 |
| [78] | G. Arenas, F. Guzman, C. Cardenas, L. Mercado and S. H. Marshall, "A novel antifungal peptide designed from the primary structure of a natural antimicrobial peptide purified from Argopecten purpuratus hemocytes", Peptides, vol. 30, no. 8, pp. 1405-11, 2009 |
| [79] | E. Tapia, C. Montes, P. Rebufel, A. Paradela, H. Prieto and G. Arenas, "Expression of an optimized Argopecten purpuratus antimicrobial peptide in E. coli and evaluation of the purified recombinant protein by in vitro challenges against important plant fungi", Peptides, vol. 32, no. 9, pp. 1909-16, 2011 |
| [80] | Ji Hyeong Baek and Si Hyeock Lee, "Isolation and molecular cloning of venom peptides from Orancistrocerus drewseni (Hymenoptera: Eumenidae)", Toxicon, vol. 55, no. 4, pp. 711-718, 2010 |
| [81] | N. Maeda and N. Tamiya, "Three neurotoxins from the venom of a sea snake Astrotia stokesii, including two long-chain neurotoxic proteins with amidated C-termini", The Biochemical Journal, vol. 175, no. 2, pp. 507-17, 1978 |
| [82] | C. N. Park, J. M. Lee, D. Lee and B. S. Kim, "Antifungal activity of valinomycin, a peptide antibiotic produced by Streptomyces sp. Strain M10 antagonistic to Botrytis cinerea", Journal of Microbiology and Biotechnology, vol. 18, no. 5, pp. 880-4, 2008 |
| [83] | A. L. Varnier, L. Sanchez, P. Vatsa, L. Boudesocque, A. Garcia-Brugger, F. Rabenoelina, A. Sorokin, J. H. Renault, S. Kauffmann, A. Pugin, C. Clement, F. Baillieul and S. Dorey, "Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine", Plant, Cell & Environment, vol. 32, no. 2, pp. 178-193, 2009 |
| [84] | Marco Kruijt, Ha Tran and Jos M. Raaijmakers, "Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267", Journal of Applied Microbiology, vol. 107, no. 2, pp. 546-556, 2009 |
| [85] | S. Joshi, C. Bharucha and A. J. Desai, "Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B", Bioresource Technology, vol. 99, no. 11, pp. 4603-8, 2008 |
| [86] | Y. Toure, M. Ongena, P. Jacques, A. Guiro and P. Thonart, "Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple", Journal of Applied Microbiology, vol. 96, no. 5, pp. 1151-60, 2004 |
| [87] | C. H. Liu, X. Chen, T. T. Liu, B. Lian, Y. Gu, V. Caer, Y. R. Xue and B. T. Wang, "Study of the antifungal activity of Acinetobacter baumannii LCH001 in vitro and identification of its antifungal components", Applied Microbiology and Biotechnology, vol. 76, no. 2, pp. 459-66, 2007 |
| [88] | Y. S. Kim, H. M. Kim, C. Chang, I. C. Hwang, H. Oh, J. S. Ahn, K. D. Kim, B. K. Hwang and B. S. Kim, "Biological evaluation of neopeptins isolated from a Streptomyces strain", Pest Management Science, vol. 63, no. 12, pp. 1208- 14, 2007 |
| [89] | M. Ubukata, M. Uramoto, J. Uzawa and K. Isono, "Structure and biological activity of neopeptins A, B, and C, inhibitors of fungal cell wall glycan synthesis", Agricultural and Biological Chemistry, vol. 50, pp. 357-365, 1986 |
| [90] | Vivek Bajpai, Hak Kim, Ching Hou and Sun Kang, "Microbial conversion and in vitro and in vivo antifungal assessment of bioconverted docosahexaenoic acid (bDHA) used against agricultural plant pathogenic fungi", Journal of Industrial Microbiology & Biotechnology, vol. 36, no. 5, pp. 695-704, 2009 |
| [91] | B. Teichmann, U. Linne, S. Hewald, M. A. Marahiel and M. Bolker, "A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis", Molecular Microbiology, vol. 66, no. 2, pp. 525-33, 2007 |
| [92] | A. Mendez-Bravo, C. Calderon-Vazquez, E. Ibarra-Laclette, J. Raya-Gonzalez, E. Ramirez-Chavez, J. Molina-Torres, A. A. Guevara-Garcia, J. Lopez-Bucio and L. Herrera-Estrella, "Alkamides activate jasmonic acid biosynthesis and signaling pathways and confer resistance to Botrytis cinerea in Arabidopsis thaliana", PLoS ONE, vol. 6, no. 11, pp. e27251, 2011 |
| [93] | Y. Brotman, A. Makovitzki, Y. Shai, I. Chet and A. Viterbo, "Synthetic ultrashort cationic lipopeptides induce systemic plant defense responses against bacterial and fungal pathogens", Applied and Environmental Microbiology, vol. 75, no. 16, pp. 5373-9, 2009 |
| [94] | T. R. Costa, O. F. Fernandes, S. C. Santos, C. M. Oliveira, L. M. Liao, P. H. Ferri, J. R. Paula, H. D. Ferreira, B. H. Sales and M. do R. Silva, "Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil", Journal of Ethnopharmacology, vol. 72, no. 1-2, pp. 111-7, 2000 |
| [95] | J. P. Benner, "Pesticidal compounds from higher plants", Pesticide Science, vol. 39, pp. 95-102, 1993 |
| [96] | M. E. Guynot, A. J. Ramos, L. Seto, P. Purroy, V. Sanchis and S. Marin, "Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products", Journal of Applied Microbiology, vol. 94, no. 5, pp. 893-9, 2003 |
| [97] | D. Kalemba and A. Kunicka, "Antibacterial and antifungal properties of essential oils", Current Medicinal Chemistry, vol. 10, no. 10, pp. 813-29, 2003 |
| [98] | C. Bouchra, M. Achouri, L. M. Idrissi Hassani and M. Hmamouchi, "Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr", Journal of Ethnopharmacology, vol. 89, no. 1, pp. 165-9, 2003 |
| [99] | Emine Mine Soylu, ≈ûener Kurt and Soner Soylu, "In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea", International Journal of Food Microbiology, vol. 143, no. 3, pp. 183-189, 2010 |
| [100] | S. Behnam, M. Farzaneh, M. Ahmadzadeh and A. S. Tehrani, "Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens", Communications in Agricultural and Applied Biological Sciences, vol. 71, no. 3 Pt B, pp. 1321-6, 2006 |
| [101] | I. Camele, V. De Feo, L. Altieri, E. Mancini, L. De Martino and G. Luigi Rana, "An attempt of postharvest orange fruit rot control using essential oils from Mediterranean plants", Journal of Medicinal Food, vol. 13, no. 6, pp. 1515-23, 2010 |
| [102] | I. Camele, L. Altieri, L. De Martino, V. De Feo, E. Mancini and G. L. Rana, "In vitro control of post-harvest fruit rot fungi by some plant essential oil components", International Journal of Molecular Sciences, vol. 13, no. 2, pp. 2290-300, 2012 |
| [103] | C. Romagnoli, R. Bruni, E. Andreotti, M. K. Rai, C. B. Vicentini and D. Mares, "Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L", Protoplasma, vol. 225, no. 1-2, pp. 57-65, 2005 |
| [104] | N. Tabanca, B. Demirci, S. L. Crockett, K. H. Baser and D. E. Wedge, "Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils", Journal of Agricultural Food Chemistry, vol. 55, no. 21, pp. 8430-5, 2007 |
| [105] | G. Wenqiang, L. Shufen, Y. Ruixiang and H. Yanfeng, "Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide", Natural Product Research, vol. 20, no. 11, pp. 992-8, 2006 |
| [106] | N. Tabanca, E. Bedir, D. Ferreira, D. Slade, D. E. Wedge, M. R. Jacob, S. I. Khan, N. Kirimer, K. H. Baser and I. A. Khan, "Bioactive constituents from Turkish Pimpinella species", Chemistry & Biodiversity, vol. 2, no. 2, pp. 221-32, 2005 |
| [107] | T. Sekine, M. Sugano, A. Majid and Y. Fujii, "Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs", Journal of Chemical Ecology, vol. 33, no. 11, pp. 2123-32, 2007 |
| [108] | S. Peighami-Ashnaei, M. Farzaneh, A. Sharifi-Tehrani and K. Behboudi, "Effect of essential oils in control of plant diseases", Communications in Agricultural and Applied Biological Sciences, vol. 74, no. 3, pp. 843-7, 2009 |
| [109] | Vivek K. Bajpai, Savita Shukla and Sun Chul Kang, "Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L", Bioresource Technology, vol. 99, no. 18, pp. 8903-8908, 2008 |
| [110] | E. K. Kulakiotu, C. C. Thanassoulopoulos and E. M. Sfakiotakis, "Biological Control of Botrytis cinerea by Volatiles of 'Isabella' Grapes", Phytopathology, vol. 94, no. 9, pp. 924-31, 2004 |
| [111] | E. K. Kulakiotu, C. C. Thanassoulopoulos and E. M. Sfakiotakis, "Postharvest Biological Control of Botrytis cinerea on Kiwifruit by Volatiles of[Isabella] Grapes", Phytopathology, vol. 94, no. 12, pp. 1280-1285, 2002 |
| [112] | K. Kishimoto, K. Matsui, R. Ozawa and J. Takabayashi, "Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana", Phytochemistry, vol. 67, no. 14, pp. 1520-1529, 2006 |
| [113] | K. Kishimoto, K. Matsui, R. Ozawa and J. Takabayashi, "Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea", Phytochemistry, vol. 69, no. 11, pp. 2127- 2132, 2008 |
| [114] | S. O. Lee, H. Y. Kim, G. J. Choi, H. B. Lee, K. S. Jang, Y. H. Choi and J. C. Kim, "Mycofumigation with Oxyporus latemarginatus EF069 for control of postharvest apple decay and Rhizoctonia root rot on moth orchid", Journal of Applied Microbiology, vol. 106, no. 4, pp. 1213-9, 2009 |
| [115] | Heidi Schalchli, Emilio Hormazabal, Jose Becerra, Michael Birkett, Marysol Alvear, Jorge Vidal and Andrés Quiroz, "Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi", Chemistry and Ecology, vol. 27, no. 6, pp. 503-513, 2011 |
| [116] | R. Huang, G. Q. Li, J. Zhang, L. Yang, H. J. Che, D. H. Jiang and H. C. Huang, "Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia", Phytopathology, vol. 101, no. 7, pp. 859-69, 2011 |
| [117] | R. Huang, H.J. Che, J. Zhang, L. Yang, D.H. Jiang and G.Q. Li, "Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits", Biological Control, vol. 62, no. 1, pp. 53-63, 2012 |
| [118] | H. M. Liu, J. H. Guo, Y. J. Cheng, P. Liu, C. A. Long and B. X. Deng, "Inhibitory activity of tea polyphenol and Hanseniaspora uvarum against Botrytis cinerea infections", Lettetters in Applied Microbiology, vol. 51, no. 3, pp. 258-63, 2010 |
| [119] | H. J. Kim, H. J. Suh, C. H. Lee, J. H. Kim, S. C. Kang, S. Park and J. S. Kim, "Antifungal activity of glyceollins isolated from soybean elicited with Aspergillus sojae", Journal of Agricultural and Food Chemistry, vol. 58, no. 17, pp. 9483-7, 2010 |
| [120] | X. J. Li, Q. Zhang, A. L. Zhang and J. M. Gao, "Metabolites from Aspergillus fumigatus, an Endophytic Fungus Associated with Melia azedarach, and Their Antifungal, Antifeedant, and Toxic Activities", Journal of Agricultural and Food Chemistry, vol. 60, no. 13, pp. 3424-31, 2012 |
| [121] | K. Kojima, Y. S. Bahn and J. Heitman, "Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans", Microbiology, vol. 152, no. Pt 3, pp. 591-604, 2006 |
| [122] | K. Yamashita, A. Shiozawa, S. Watanabe, F. Fukumori, M. Kimura and M. Fujimura, "ATF-1 transcription factor regulates the expression of ccg-1 and cat-1 genes in response to fludioxonil under OS-2 MAP kinase in Neurospora crassa", Fungal Genetics and Biology, vol. 45, no. 12, pp. 1562-9, 2008 |
| [123] | D. Hagiwara, Y. Asano, J. Marui, A. Yoshimi, T. Mizuno and K. Abe, "Transcriptional profiling for Aspergillusnidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress", Fungal Genetics and Biology, vol. 46, no. 11, pp. 868-78, 2009 |
| [124] | R. Noguchi, S. Banno, R. Ichikawa, F. Fukumori, A. Ichiishi, M. Kimura, I. Yamaguchi and M. Fujimura, "Identification of OS-2 MAP kinase-dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa", Fungal Genetics and Biology, vol. 44, no. 3, pp. 208-18, 2007 |
| [125] | R. R. Lew, "Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora crassa", Fungal Genettics and Biology, vol. 47, no. 8, pp. 721-6, 2010 |
| [126] | N. Ochiai, M. Fujimura, T. Motoyama, A. Ichiishi, R. Usami, K. Horikoshi and I. Yamaguchi, "Characterization of mutations in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa", Pest Management Science, vol. 57, no. 5, pp. 437-42, 2001 |
| [127] | S. Y. Kim, Y. J. Ko, K. W. Jung, A. Strain, K. Nielsen and Y. S. Bahn, "Hrk1 plays both Hog1-dependent and -independent roles in controlling stress response and antifungal drug resistance in Cryptococcus neoformans", PLoS ONE, vol. 6, no. 4, pp. e18769, 2011 |
| [128] | J. H. Kim, B. C. Campbell, N. Mahoney, K. L. Chan, R. J. Molyneux and G. S. May, "Enhancement of fludioxonil fungicidal activity by disrupting cellular glutathione homeostasis with 2,5-dihydroxybenzoic acid", FEMS Microbiology Letters, vol. 270, no. 2, pp. 284-90, 2007 |
| [129] | J. H. Kim, B. C. Campbell, N. Mahoney, K. L. Chan, R. J. Molyneux and G. S. May, "Enhanced activity of strobilurin and fludioxonil by using berberine and phenolic compounds to target fungal antioxidative stress response", Letters in Applied Microbiology, vol. 45, no. 2, pp. 134-41, 2007 |
| [130] | J. H. Kim, B. C. Campbell, N. Mahoney, K. L. Chan, R. J. Molyneux and C. L. Xiao, "Use of chemosensitization to overcome fludioxonil resistance in Penicillium expansum", Letters in Applied Microbiology, vol. 51, no. 2, pp. 177-83, 2010 |
| [131] | J. Heller, N. Ruhnke, J. Espino, M. Massaroli, I. G. Collado and P. Tudzynski, "The MAP kinase BcSak1 of Botrytis cinerea is required for pathogenic development and has broad regulatory functions beyond stress response", Molecular Plant-Microbe Interactions, vol. 25, no.5, pp. 802-16, 2012 |
| [132] | Nora Temme, Birgitt Oeser, Michelli Massaroli, Jens Heller, Adeline Simon, Isidro Gonzalez Collado, Muriel Viaud and Paul Tudzynski, "BcAtf1, a global regulator, controls various differentiation processes and phytotoxin production in Botrytis cinerea", Molecular Plant Pathology, vol. 13, no. 7, pp. 704-18, 2012 |
| [133] | S. Giesbert, J. Schumacher, V. Kupas, J. Espino, N. Segmueller, I. Haeuser-Hahn, P. Schreier and P. Tudzynski, "Identification of pathogenesis associated genes by T-DNA-mediated insertional mutagenesis in Botrytis cinerea: a type 2A phosphoprotein phosphatase and a SPT3 transcription factor have significant impact on virulence", Molecular Plant-Microbe Interactions, vol. 25, no. 4, pp. 481-95, 2011 |
| [134] | G. Loebenstein, "Local lesions and induced resistance", Advances in Virus Research, vol. 75, pp. 73-117, 2009 |
| [135] | G. Loebenstein, D. R. David, D. Leibman, A. Gal-On, R. Vunsh, H. Czosnek and Y. Elad, "Tomato plants transformed with the inhibitor-of-virus-replication gene are partially resistant to Botrytis cinerea", Phytopathology, vol. 100, no. 3, pp. 225-9, 2010 |
| [136] | A. Dehestani, K. Kazemitabar, G. Ahmadian, N. B. Jelodar, A. H. Salmanian, M. Seyedi, H. Rahimian and S. Ghasemi, "Chitinolytic and antifungal activity of a Bacillus pumilus chitinase expressed in Arabidopsis", Biotechnology Letters, vol. 32, no. 4, pp. 539-46, 2010 |
| [137] | S. Koskela, P. P. Soderholm, M. Ainasoja, T. Wennberg, K. D. Klika, V. V. Ovcharenko, I. Kylanlahti, T. Auerma, J. Yli-Kauhaluoma, K. Pihlaja, P. M. Vuorela and T. H. Teeri, "Polyketide derivatives active against Botrytis cinerea in Gerbera hybrida", Planta, vol. 233, no. 1, pp. 37-48, 2011 |
| [138] | S. Brauc, E. De Vooght, M. Claeys, J. M. Geuns, M. Hofte and G. Angenon, "Overexpression of arginase in Arabidopsis thaliana influences defence responses against Botrytis cinerea", Plant Biology, vol. 14 Suppl 1, no. pp. 39-45, 2012 |
| [139] | S. I. Kirubakaran, S. M. Begum, K. Ulaganathan and N. Sakthivel, "Characterization of a new antifungal lipid transfer protein from wheat", Plant Physiology and Biochemistry, vol. 46, no. 10, pp. 918-27, 2008 |
| [140] | G. Corrado, P. D. Bovi, R. Ciliento, L. Gaudio, A. Di Maro, S. Aceto, M. Lorito and R. Rao, "Inducible Expression of a Phytolacca heterotepala Ribosome-Inactivating Protein Leads to Enhanced Resistance Against Major Fungal Pathogens in Tobacco", Phytopathology, vol. 95, no. 2, pp. 206-15, 2005 |
| [141] | Z. Zheng, A. Qualley, B. Fan, N. Dudareva and Z. Chen, "An important role of a BAHD acyl transferase-like protein in plant innate immunity", The Plant Journal, vol. 57, no. 6, pp. 1040-53, 2009 |
| [142] | L. Saiz-Urra, A. J. Bustillo Perez, M. Cruz-Monteagudo, C. Pinedo-Rivilla, J. Aleu, R. Hernandez-Galan and I. G. Collado, "Global antifungal profile optimization of chlorophenyl derivatives against Botrytis cinerea and Colletotrichum gloeosporioides", Journal of Agricultural and Food Chemistry, vol. 57, no. 11, pp. 4838-43, 2009 |
| [143] | H. J. Kim, H. J. Park and S. H. Choi, "Antimicrobial action effect and stability of nanosized silica hybrid Ag complex", Journal of Nanoscience and Nanotechnology, vol. 11, no. 7, pp. 5781-7, 2011 |
| [144] | Dawn Worrall, Geoff H. Holroyd, Jason P. Moore, Marcin Glowacz, Patricia Croft, Jane E. Taylor, Nigel D. Paul and Michael R. Roberts, "Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens", The New Phytologist, vol. 193, no. 3, pp. 770-778, 2012 |
| [145] | J. Pham, J. Liu, M. H. Bennett, J. W. Mansfield and R. Desikan, "Arabidopsis histidine kinase 5 regulates salt sensitivity and resistance against bacterial and fungal infection", The New Phytologist, vol. 194, no. 1, pp. 168-80, 2012 |
| [146] | T. Shindo, J. C. Misas-Villamil, A. C. Horger, J. Song and R. A. van der Hoorn, "A role in immunity for Arabidopsis cysteine protease RD21, the ortholog of the tomato immune protease C14", PLoS ONE, vol. 7, no. 1, pp. e29317, 2012 |
| [147] | R. Guetsky, D. Shtienberg, Y. Elad and A. Dinoor, "Combining biocontrol agents to reduce the variability of biological control", Phytopathology, vol. 91, no. 7, pp. 621-7, 2001. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML