-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(5): 120-126
doi: 10.5923/j.ijmb.20120205.01
Phytochemical Composition of Costus Afer Extract and Its Alleviation of Carbon Tetrachloride – induced Hepatic Oxidative Stress and Toxicity
Ukpabi Chibueze F.1, Agbafor Kingsley N.2, Ndukwe Okorie K.3, Agwu Akuagwu4, Nwachukwu Success N.4
1Department of Biochemistry, Abia State Polytechnic, Aba, Abia State Nigeria
2Department of Biochemistry, Ebonyi State University, Abakiliki, Ebonyi State Nigeria
3Department of Biology, Abia State Polytechnic, Aba, Abia State Nigeria
4Department of Chemistry, Abia State Polytechnic, Aba, Abia State Nigeria
Correspondence to: Ukpabi Chibueze F., Department of Biochemistry, Abia State Polytechnic, Aba, Abia State Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Medicinal plants contain bioactive compounds capable of preventing and fighting oxidative related diseases. Phytochemical composition of costus afer extract and its alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity were evaluated. The qualitative and quantitative analyses showed the presence of alkaloids, saponins, flavonoids, tannins and phenols in the aqueous stem extract while flavonoids, saponins and phenols were detected in the ethanol extract. The toxicological study showed serum elevation of alanine aminotransferase (ALT, EC 2.6.1.2.), aspartate aminotransferase (AST, EC 2.6.1.1) and alkaline phosphatase (ALP, EC 3.1.3.1) and increase in the levels of thiobarbituric acid reactive species (TBARS) expressed as melondialdehyde in the liver of the rats in responds to the oral administration of carbon tetrachloride. The rats fed with 400mg/kg of extract of costus afer showed significant (p<0.05) reduction in ALT and ALP levels close to the control than the rats fed with 200mg/kg. Though there were reduction in AST and TBARS levels close to the control than the rats fed with 200mg/kg, they were not significant (p>0.05). This indicated that biological active compounds of costus afer are more polar and could serve as source of bioactive compounds for nutrition and therapeutic purposes.
Keywords: Costus Afar, Phytochemicals, Carbon Tetra Chloride and Hepatotoxicity
Cite this paper: Ukpabi Chibueze F., Agbafor Kingsley N., Ndukwe Okorie K., Agwu Akuagwu, Nwachukwu Success N., "Phytochemical Composition of Costus Afer Extract and Its Alleviation of Carbon Tetrachloride – induced Hepatic Oxidative Stress and Toxicity", International Journal of Modern Botany, Vol. 2 No. 5, 2012, pp. 120-126. doi: 10.5923/j.ijmb.20120205.01.
Article Outline
1. Introduction
- In Nigeria many indigenous plants are used as spices, food or medicine. These plants often exhibit a wide range of biological and pharmacological activities. Extracts from the roots, backs, seeds and fruits of these plants are used in the preparation of syrups in traditional medicine as cough suppressarant and in the treatment of oxidative related diseases[12].It is generally assumed that the active constituents contributing to these protection effects are the phytochemical constituents, vitamins and minerals[12]. Phytochemical constituents exhibit a wide range of biological effects resulting in their protective or disease preventive properties. These are some possible actions: antioxidant, hormonal action, stimulation of enzymes, interference with DNA replication, anti-microbial effect, and physical action[20][13][25].Costus afer. Ker (family; zingiberaceae) is a tall perennial herbaceous, unbranched medicinal plant with creeping rhizome. It can be found in shady forest and riverbanks of Senegal, South Africa, Guinea, Nigeria, Ghana and Cameroon[3]. The plant is commonly known as gingerilily. It is known as “Okpete” or “Okpoo” in Igboland, “Kakizawa” in Hause, “tete-egun” in Yoruba and “Mbritem” in Efik, all in Nigeria. Anglophone Cameroon calls it “Monkey sugar cane”.[26]An ethnobotanical survey of the genus costus revealed that the methanolic leaf extract of costus afer contains potent biological active compounds, which have moderate agonistic properties on guinea pig ileum, abortificient property at 3rd trimester of pregnancy and antihyperglycermic property on streptozotocin-induced hyperglycermia[26][27]. Additionally, methanol extracts of costus pictus D. Don ex Lindl. lowered blood glucose concentrations and increased plasma insulin concentration in alloxan- induced diabetic rats[28]. The rich steroidal saponins and sapogenic constituents, mainly simple phenolics and cyanogenic glycosides may be responsible for the antidiabetic properties[29]. However, costus spicatus tea failed to improve diabetic progression in C57BLKS/J db/db mile, a model or type 2 diabetes mellitus[30]. Furthermore, methanol extract of costus afer leaf showed significant antipyretic potential in normal body temperature and brewer’s yeast induced pyrexia in albino rats[31].In toxicological studies of acute exposure, changes in concentrations and enzyme activities often directly reflect cell and tissue damage in specific organs[22]. Carbon tetrachloride (CCl4) is known to affect almost every functional site of animal tissues, often due to their bioaccumulation and poor excretion[11]. Alanine aminotransferase (ALT, EC 2.6.1.2) and aspartate aminotransferase (AST, EC 2.6.1.1), as well as alkaline phosphatase (ALP, EC 3.1.3.1.) participate in transamination and dephosphorylation reactions and are found predominantly in liver, cardiac cells and striated muscle tissues[7]. Recent studies have pointed out that CCl4 hepatotoxicity is associated with the formation of free radicals, which may cause severe injury damage to tissues system[14]. Base on the above studies, we hypothesize that this hepatotoxic substance could also accumulate in the liver to toxic levels and cause pathological alterations. Cellular damage release ALT, AST and ALP into the blood stream and the levels of these enzymes have the potential to indicate hepatotoxicity[4]. Lipid peroxidation is an autocatalytic process which could cause necrosis in various tissues in the body. The measurement of thiobarbituric acid reactive species (TBARS) has been the method of choice for measuring lipid peroxidation, an indicator of oxidative stress[11]. The extent of organ damage is dependent on the type of toxicant, its mechanism of action and duration of exposure[22].
1.1. Objectives
- Due to Costus afer current use in some communities for treating varieties of chronic diseases, it is important to further study this herb and its potential utility in the management of hepatotoxicities. Oxidative stress and damage by free radicals to biomolecules have been linked to the pathology of a variety of chronic diseases. Medicinal plants contain bioactive compounds capable of preventing and fighting oxidative related diseases. These compounds must be screened and assayed before effective drugs are developed. Costus afer could be a promising plant for the development of anti-hepatitic drugs. The aim of this paper is to evaluate the phytochemical constituents of Costus afer extract and its alleviation of carbon tetrachloride induced hepatic oxidative stress and toxicity.
2. Material and Method
2.1. Chemicals and Equipments
- Carbon tetrachloride (CCl4) was obtained from BDH chemicals (Poole, England). All other chemicals and reagents were of analytical grade. Optical densities were measured using digital spectrophotometer (Model 390, Tuner® USA)
2.1.1. Plant Material
- The stems of costus afer were collected fresh from Ogbor Hill riverbank in Aba, Abia State Nigeria in July, 2011. This was identified by the natives as “Okpete” and Voucher specimens of the plants were deposited in Herbarium, Department of Plant Science and Biotechnology, Michael Okpara University of Agriculture Umudike, Abia State Nigeria, after proper identification.
2.1.2. Animals
- Twenty albino rats of the wister strain of both sexes weighting 80-100g were purchased from laboratory animal unit of the Department of Biochemistry, Federal University of Technology Owerri Nigeria. The animals were kept in a separate animal room on a 12 hour light/dark cycle at a room temperature of 22±2℃ and with free access to food (Guinea feed® Benin City, Nigeria) and water at libitum. They were acclimatized to laboratory conditions for six days and housed in steel cages. All the animals were starved overnight before experimentation[19].
2.1.3. Qualitative Phytochemical Analysis
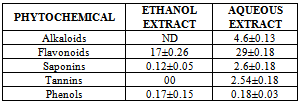
- Phytochemical screening was performed on the extracts using standard procedures to indentify chemical constituents as described by Harborne[6] and Sofowora[17].AlkaloidThe aqueous and ethanol extracts were evaporated to dryness and the residue (0.5g) was stirred in 5 ml of 1% HCl on a steam bath and filtered while hot. Distilled water was added to the residue and 1 ml of the filtrate was treated with a few drops of Wagner’s reagent. A reddish brown precipitate indicates the presence of alkaloids.FlavonoidTwo milliliters of dilute sodium hydroxide was added to 2 ml of the extract. The appearance of a yellow colour indicates the presence of flavonoids.PhenolsEqual volumes (1 ml) of extracts and iron(III)chloride were mixed. A deep bluish green solution gave an indication of the presence of phenols.TanninsA portion of the extract was dissolved in water, after which the solution was clarified by filtration 10% ferric chloride solution was then added to the resulting filtrate. The appearance of a bluish black colour indicates the presence of tannins.
2.1.4. Quantitative Phytochemical Analysis
- Alkaloids ContentThis was done by the alkaline precipitation gravimetric method of Harborne[6], as described by Obadoni and Ochuko[10] and Okwu[12]. Each of the samples (5g) was weighed into a 250ml beaker and 200ml of 20% acetic acid in ethanol (1:10) was added and covered to stand for 4hrs. This was filtered and the extract was concentrated using water –bath to one-quarter of its original volume by evaporation and treated with drop-wise addition of conc. aqueous NH4OH until alkaloids was precipitated. The whole solution was allowed to settle and the precipitate was collected by filtration which was later dried and weighed. The alkaloid content was calculated and expressed as mg/100g of the weight samples analyzed.Tannins ContentTannin content was determined by the photometric method of Harborne[6] as described by Okwu[12]. The samples (5g) were weighed into 100ml plastic bottle. 50ml of distilled water was added and shaken for 1hr in a mechanical shaker. This was filtered into a 50ml volumetric flask and made up to the mark with distilled water. Then 5ml of the filtrate was pipette out into a tube and mixed with 3ml of 0.1M FeCl3 in 0.1N HCl and 0.008M potassium ferrocyanide. The absorbance was measured in a spectrophotometer at 760nm wavelengths, within 10 minutes. A blank sample was prepared using the extraction solution (distilled water). A standard solution of tannic acid (5mg/ml) was prepared and with the blank reagent at zero the standard absorbance was measure at 760mm wavelength. Saponins ContentThis was done by the method of Harborne[6] as described by Obadoni and Ochuko[10], and Okwu[12]. Each of the plant samples (5g) were dispersed in 200ml of 20% ethanol. The suspension was heated over a hot water bath for 4hrs with continuous stirring at about 55℃. The mixture was filtered and the residue re-extracted with another 200ml of 20% ethanol. The combined extracts were reduced to 40ml over a water bath at about 90℃. The concentration was transferred into a 250ml separator funnel and 20ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated after which n-butanol (60ml) was subsequently added and the combined n-butanol extracts were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution was poured into a dried pre-weighed crucible and dried in an oven at 60℃ to a constant weight. Flavonoids ContentThe total flavonoid content of plant extract was determined using Harborne as described by Okwu[13]. Each of the plant samples (5g) was weighted into 100ml plastic bottle and extracted repeatedly with 100ml of 80% aqueous ethanol at room temperature. It was filtered with Whatman No 42 fitter paper into 100ml flask. The filtrate was transferred into a crucible dish and evaporated to dryness over a water bath. This was further dried in an oven at 60℃ at 30 minutes and cooled in desiccators. The crucible and the content were weighted and recorded. Total Phenolic ContentTotal phenol content was determined using the Harborne method[6] as described by Obadoni and Ochuko[10], and Okwu[12]. For the extraction of the phenolic component, the fat free sample was boiled with 50ml of ether for 15 minutes. The extract (5ml) was pipette into a 50ml flask, and 10ml of distilled water was added. 2ml of ammonium hydroxide solution and 5ml of concentration amyl alcohol were also added. The samples were left to react for 30 minutes for colour development. The absorbance of the solution was read using a spectrophotometer at 505nm wavelength. A blank sample for each extract was used for background subtraction. A standard phenol was prepared as 0.05mg/l and absorbance measured. The total phenolic content was expressed as mg/100g.
2.1.5. Animal Treatment
- The rats were split into four groups: group 1, 2, 3, and 4. the aqueous costus afer extract was orally given to rats in 200mg/kg body weight and 400 mg/kg body weight respectively. The rats in group 1 were untreated control while rats in group 2 were only treated with carbon tetrachloride. The carbon tetrachloride was mixed with olive oil at the ratio of 1:1 w/v and was orally given at 1 ml/kg body weight. Twenty four hours after the administration of the carbon tetrachloride, rats in group 3 and 4 were given the costus after extract orally once daily for six days. Throughout the experiment, the rats were allowed unlimited access to livestock feed for rats and drinking water at libitum.
2.1.6. Blood and Tissue Collection
- Twenty four hours after the last treatment, each rat was subjected to light anaesthesis in a diethyl ether saturated chamber and dissected. The thoracic region was opened and blood drawn through direct cardiac punctures and delivered into sterile sample bottles having no anticoagulant. Blood was allowed to stand for 10min and centrifuged for 10 mins of 4000 rpm in order to collect serum. The liver was excised immediately and washed in ice cold phosphate buffer (0.2M, pH 7.0). A 10% tissue homogenate was prepared using the same buffer and perinuclear fraction was obtained by centrifuging homogenate at 4000 rpm for 30 mins at 4℃.
2.1.7. Biochemical Assay
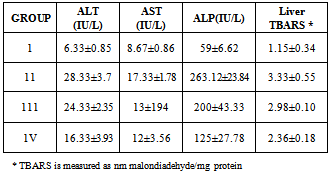
- Determination of Biochemical Parameters in serumThe activities of alanine aminotransferase (ALT, EC 2.6.1.2) and aspartate aminotransferase (AST, EC 2.6.1.1) was measured in serum according to Reitman and Frankel[24]. Alkaline phosphatase (ALP, EC 3.1.3.1) were also determined in serum according to the instructions on the diagnostic kits purchased from Randox laboratories (UK). Thiobarbituric acid reactive substances (TBARS) assayLipid peroxidation in the supernatant fractions was determined spectrophotometrically by assessing the concentration of thiobarbituric acid reactive substances (TBARS) as described by Liu[23].The results were expressed in Malondiadehyde formed relative to an extinction coefficient of 1.56 x 106 mol/cm. One milliliter of the perinuclear fraction of each tissue was incubated with 2ml of the thiobarbituric acid reagent (0.37% w/v thiobarbituric acid, 50% v/v conc. hydrochloric acid, and 5% trichloroacetic acid). The solution was boiled for 15 min. it was cooled and later centrifuged at 5000 rpm for 5 mins. The absorbance of the supernatant was later read at 532 nm. TBARS was expressed as nanomoles of malondiadehyde per milligram protein.
3. Result
- The qualitative and quantitative phytochemical analyses of aqueous and ethanol extract of c. afer are presented in Tables 1 and 2 respectively. The result indicated that the phytochemical constituents were abundant in aqueous extract than the ethanol extract. The activities of ALT, AST, ALP and liver levels of TBARS of rats treated with carbon tetrachloride are presented in Table 3. It was observed that when the rats were orally treated with carbon tetrachloride alone, the levels of the markers were significantly higher than control (p<0.05). The levels which were elevated were reduced close to control levels when the Costus afer etract was orally given to the rats prior to the administration of carbon tetra chloride as shown in Table 3. The figures 1 and 2 represent the plot of Phytochemical composition (mg/100g) of aqueous and ethanol Extracts of Costus afer and Carbon tetra chloride induced alterations on blood variables and liver levels of TBARS .
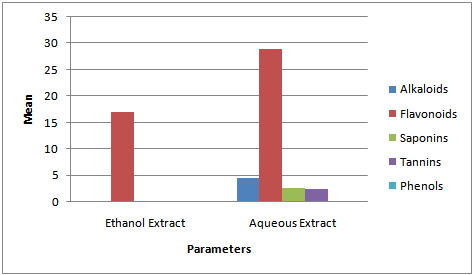 | Figure 1. Phytochemical composition (mg/100g) of Aqueous and Ethanol Extracts of Costus Afer |
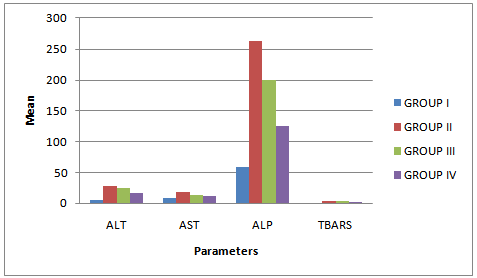 | Figure 2. Effect of Costus Afer Extract on Carbon tetra chloride induced alterations on blood variables and liver levels of TBARS |
4. Discussion
- The present study investigated the phytochemical constituents of aqueous and ethanol extracts of costus afer. The qualitative phytochemical analysis of the stem extract showed the presence of alkaloids, saponins, flavonoids tannins and phenols in the aqueous extract while flavonoid, saponins and phenols were detected in the ethanol extract. These screened phytochemical constituents were showed to be highly soluble in water. Alkaloids are known to have anti-inflammatory, antifungal and anti-microbial effects[13] as well as anti-hypertensive agent[17] while tannins have been found to have healing effect[13]. Flavonoids, saponins, and phenols were abundant in aqueous extract than ethanol extract. These are potent water soluble antioxidants which prevent oxidizing cell damage suggesting anti-inflammatory properties. The therapeutic potential of antioxidants in controlling degenerative disease with marked oxidative damage from reactive oxygen species or free radicals have been reported[1],[18],[21]. From the quantitative analysis, aqueous extract of costus afer showed significant (p< 0.05) high content of flavonoids, and saponins than ethanol extract. Aqueous extract showed the quantity of flavoniods and saponins as 29+0.18 mg/100g and 2.6 +0.10mg/100g while ethanol extract showed 17.0+0.2.6 and 0.12+ 0.05mg/100g respectively. Previous studies have shown that alkaloids are more soluble in water than in other organic solvent[1]. This is therefore an indication that the biological active compounds of costus afer are more polar and as such are contained in the aqueous extraction of the stemsThe elevation of the levels of ALT, AST and ALP in response to the oral administrating of carbontetrachloride showed its hepatotoxic nature which is in accordance with previous studies[11]. The toxicity of carbon tetrachloride may require the activation to a highly -reactive metabolite via reactions catalysed by cytochrome p450[16],[5]. Early report concerning the its pathological effect of Carbontetrachloride showed that the mitochondria were altered after carbontetrachloride poisoning of rats[16]. This toxicity of carbon tetra chloride has been linked to the production of free radicals. This is further supported by the increase in malondialdehyde in the liver of the rats treated with carbontetrachloride only. Lipid peroxideation is an autocatalytic process which could cause necrosis in various tissues in the body. The measurement of TBARS has been the method of choice for measuring liquid peroxidation, an inidicator of oxidative stress[11]. Cells basically have the capacity to tolerate free radicals/reactive oxygen species since it is a basic feature of metabolism. This is as a result of the presence of various antioxidants and free radicals scavenging systems. But serious pathological consequences occur when there is an imbalance of the antioxidants/antioxidant system and the free radicals/reactive oxygen species- a condition known as oxidantive stress[11]. This condition has been shown to be the basis of oxidative related diseases[12].Liver injury is defined as an alanine aminotransferase (ALT) level of more than three times the upper limit of the normal range and an alkaline phosphatase (ALP) level of more than twice the upper limit of normal range[19]. Liver injury is further characterized as hepetocellular when there is a predominant initial elevation of the ALT level or as cholestatic when there is a predominant initial elevation of the ALP level; a mixed pattern comprises elevations of both the ALT and ALP levels. From the results obtained in this study, the rats treated with 1ml/kg body weight of the Carbon tetra chloride showed a significant elevation (p < 0.05) in ALT, AST, ALP and TBARS levels over the control levels. These elevated levels rated 4.47, 1.99, 4.45 and 2.89 times respectively higher than the control levels.Costus afer ameliorated the toxicity of carbontetrachloride, which is dose-deperodent. The rats treated with 400mg/kg of extract showed significant (p< 0.05) reduction in ALT and ALP levels close to control than the rats fed with 200mg/kg of the extractThough there were reduction in AST and TBARS levels close to the control levels than the rats fed with 200mg/kg of the extract, they were not significant (p<0.05). The reduction in the activities of the ALT, AST, ALP and liver TBARS towards the respective normal values by plant extract is an indication of the stabilization of plasma membranes as well as the membranes of the liver tissues, caused by the carbon tetra chloride. These screened phytochemicals constituents have been shown to protect against various forms of oxidative related disease via the induction of detoxifying enzymes such as epoxide hydroxylase, glutathione s-transferase etc.[2],[8]. Although the molecular mechanism through which the induction of these detoxification enzymes is not well known, it has been supported that these phytochemeicals interact with various intracellular signaling Casceders[15].Costus afer has been shown by this study to contain phytochemical constituents which can act synergistically to reduce the toxicity induced by Carbon tetra chloride. These suggest its potential in the treatment and prevention of various oxidative related diseases. Therefore, stem extract of costus afer could be exploited as sources of free radical scavengers and bioactive metabolites for nutritional, medicinal and commercial purposes.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the technical assistance received from the staff of Yemac Consulting and Analytical Services Limited Aba, Nigeria.
Appendix
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML