-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(4): 103-107
doi: 10.5923/j.ijmb.20120204.07
Would Predatory Drillhole Frequency on Chione spp. Increase under the Suggested Climate Change Scenario? Comparing Pleistocene and Modern Rhodolith Beds
Carlos E. Cintra-Buenrostro
Dept. of Chemistry and Environmental Sciences, University of Texas at Brownsville, Brownsville, TX, 78520, USA
Correspondence to: Carlos E. Cintra-Buenrostro , Dept. of Chemistry and Environmental Sciences, University of Texas at Brownsville, Brownsville, TX, 78520, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The analysis of predatory drillholes allow paleontologists to reconstruct trophic relationships of ancient systems. To evaluate the potential effects of climate and sea level change on predation frequency by gastropods on bivalves, species of the genus Chione from the Gulf of California were selected because they are a commercially exploited resource - at least locally - and abundant in both modern and Pleistocene (sea level high stand of oxygen substage 5e) samples from rhodolith beds. The present study focused to answer a practical question: would predation on Chione spp. increase under the suggested climate - warming - change scenario? A total of 1,626 clams were examined for predatory drillholes, approximately 10.3% of these were drilled. Examination of the drillholes indicated that naticid gastropods were the main predators (~ 98.2%). Both drilling frequency and percent preyed upon were larger in modern than in fossil clams. The higher values observed in modern samples are likely due to differences in temperature between the two times as shell deposition increases with higher temperatures. The higher temperatures in the Pleistocene likely resulted in increased shell thickness, making drilling by predators more difficult.
Keywords: Mollusk, Bivalvia, Oxygen Isotope Substage 5e, Pleistocene, Global Climatic Change
Cite this paper: Carlos E. Cintra-Buenrostro , "Would Predatory Drillhole Frequency on Chione spp. Increase under the Suggested Climate Change Scenario? Comparing Pleistocene and Modern Rhodolith Beds", International Journal of Modern Botany, Vol. 2 No. 4, 2012, pp. 103-107. doi: 10.5923/j.ijmb.20120204.07.
1. Introduction
- Drilled holes through calcareous exoskeletons of prey have been attributed to predatory gastropods since the 4th century B.C. by Aristotle[1]. The analysis of predatory drillholes now allows paleontologists to reconstruct trophic relationships of ancient systems[for reviews, see 2, 3, 4, 5, 6]. These analyses have shown that the majority of modern bivalve mollusks have been drilled by naticid or muricid gastropods, although octopi, flatworms and certain nematods are also capable of drilling while preying upon bivalves[5, 7, 8, 9, 10, 11]. Furthermore the size, shape, and location of drillholes provide evidence for the identity, size, behavior, and even prey preference of drilling predators[reviewed in 12].Mollusks are the most conspicuous fauna inhabiting the southwestern Gulf of California rhodolith (unattached non-geniculate coralline algae) beds[13, 14]. Pleistocene (approximately 125,000 yrs) rhodolith beds are part of the prominent formations of shallow marine sediments deposited during the sea level high stand of oxygen substage 5e (hereafter referred as I.S. 5e[15]) along the Gulf of California coasts. To evaluate the potential effects in climate and sea level change on predation frequency by gastropods on bivalves, species of the genus Chione were selected because they are abundant in both modern and I.S. 5e samples and are a commercially exploited resource - at least locally. Although relative abundance of either I.S. 5e or modern samples can be altered by selective preservation of the I.S. 5e beds or selective harvesting from the modern beds[16, 17], the more than 1,626 individuals used in the study allow for a robust comparison.Sea level rise (SLR) is considered one of the greatest challenges of this century, particularly its socioeconomic impacts[18]. Sea level rose ~ 120 m since the last glacial maximum[19], after which time it has remained close to that of the present day. Nonetheless, global climatic warming has led to thermal expansion of the ocean resulting in a net influx of water from melting glaciers[20], which has led to several atmospheric-ocean global circulation models under various warming scenarios. The IPCC[21] estimates a SLR of 0.18 to 0.59 m by the end of this century relative to the 1980-1999 sea-level position. Although at the highest estimated rate of SLR - assuming a constant rate of sea level change - it would take up to 10 centuries to reach the approximate sea level present during I.S. 5e (~ 6 m above present[22]), the present study allows an answer to the practical question for a commercially (at least locally) exploited resource (Chione spp.): would predation on Chione spp. increase under the suggested climate - warming - change scenario? The predicted increase of ~ 2-3℃ in water temperature[23, 24, 25] or in the range of 2-4.5℃ with the best estimate of 3℃ but no less than 1.5℃[21] allows for a comparison of the I.S. 5e (when temperatures were ~ 2-3℃ warmer than today) and modern predation frequency in Chione spp.
2. Study Area and Methods
- Shells of the bivalve Chione spp. were used in the present study; identification to species level was not possible either because of lack of appropriate identifying features or damage within the I.S. 5e taxa. Chione spp. includes the sister species C. californiensis and C. undatella[26]. Modern samples were collected in Bahía Concepción (26° 39’ 02” N, 111° 49’ 08” W) in March 1997 and March 1999, and I.S. 5e samples were collected in Punta Chivato (27° 4’ N, 111° 57’ W) Baja California Sur, México[see 14 for a description of the study site].Ten random samples of modern mollusks, taken at minimum 50 m from each other, were collected with SCUBA using a cylinder with a saw-like edge buried to ~ 20 cm in the substratum. The modern samples were taken along the 3-12 m depth gradient of the studied rhodolith beds and sieved underwater through a screen of 1 cm mesh size. Each sample yielded ~ 20,000 cm3. The same number of samples and mesh screen size were used to systematically collect the I.S. 5e material, obtaining ~ half of the sieved material (~ 10,000 cm3) for each fossil sample. The difference in sieved volume between I.S. 5e and modern samples was due to the relatively lower abundance of shell and rhodolith material in the latter as explained by Cintra-Buenrostro et al.[14], who argued that standardization of units (e.g. individuals/cm3) was not appropriate based on the composition of the samples.Drilling frequency was estimated using Kowalewski’s[27] equation 5 (fd = d/0.5n) for disarticulated elements, where fd = estimated drilling frequency, d = number of valves in the sample with at least one successful drillhole, and n = total number of valves in the sample. All drillholes were measured with a caliper (precision + 0.01 mm).A two-sample t-test, α = 0.05 was used to determine if the predation percentage was different between I.S. 5e and modern samples. All data were tested for homoscedasticity[28] and no transformations were necessary. Percent predation data were not normally distributed but a two-sample t-test was still used with non-transformed data because such tests are robust to violations of the normality assumption[29].A Chi-square (χ2) was performed to test for non-randomness selectivity of a drilling site in Chione spp. using the within-element sector approach sensu Kowalewski[27]. For this purpose each shell was divided into nine uneven sectors (Fig. 1), and the presence of a drillhole in each sector was recorded. If some drillholes covered more than one sector the sector with more than 50% of the outside drillhole diameter was selected for the analysis.
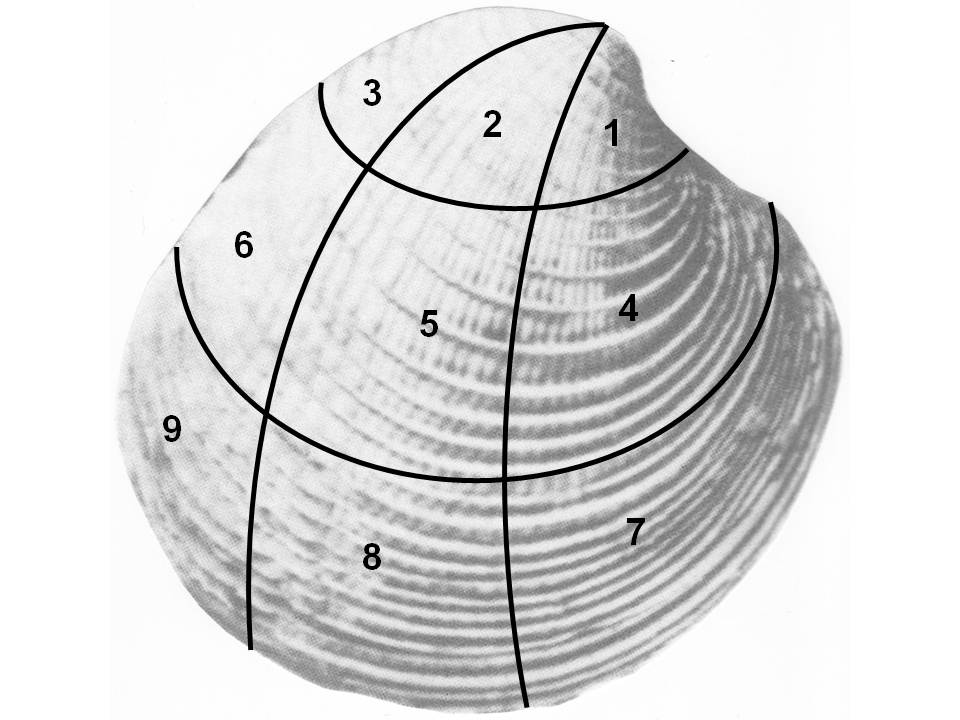 | Figure 1. Diagram of Chione spp. shell showing uneven sectors for recording gastropod drillholes site selectivity |
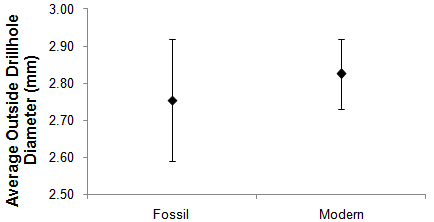 | Figure 2. Average + standard error (bars) outside drillhole diameter in Chione spp. for: 30 Fossil = I.S. 5e and 142 Modern specimens |
3. Results
- A total of 1,626 clams (by the definition a clam consist of two valves - 512 were I.S. 5e and 1,114 modern) of Chione spp. were examined for predatory drillholes. Approximately 10.3% were drilled with only 0.7% incomplete holes from the total number of clams, the other ~ 9.6% were successfully drilled. Examination of the drillholes indicated that naticid gastropods were the main predators (~ 98.2%), the presence of muricid drillholes was almost negligible, accounting for the remaining 1.8%. All muricid predation occurred in the Pleistocene I.S. 5e samples. Only four modern bivalves (2.4% of all drilled) showed evidence of more than one drillhole and in all of these cases there were two complete drillholes attributed to a naticid gastropod. Drillhole sizes were not significantly different between I.S. 5e and modern specimens (Fig. 2).The estimated average drilling frequency for all Chione spp. was 0.09 + 0.01 and was larger in modern (average 0.13 + 0.02) than in fossil (0.05 + 0.01) clams. This difference was also detected and statistically significant using percent preyed upon Chione spp. between I.S. 5e and modern samples: t (0.05) 9,9 = - 4.93, p = 0.001. Chione spp. individuals were non-randomly drilled by gastropods, mainly naticids (χ2 0.05,8 = 110.45, p > 0.05); with ~ 43.6% of the drillholes
4. Discussion
- The overwhelming predation (98.2%) by naticid gastropods is not surprising based on the difference in abundance observed by Cintra Buenrostro[30] between the two families of gastropods: Naticidae was represented by 342 individuals (330 of them in the I.S. 5e samples) while Muricidae accounted for 32 (22 of which were I.S. 5e). Although their abundance suggests that muricids should account for ~ 8.6% of the successful predation, the difference is easily explained by Chione spp., the prey species analyzed. Instead of the minimum 92 bivalves taxa potentially (number should be higher because both naticids and muricids are known to prey on other snails, and the area yielded 97 gastropod taxa as well[14, 30] available for the gastropods to prey on in the studied area. Furthermore, muricids are also known to eat carrion[31].The overwhelming abundance of I.S. 5e gastropods also helps explain the observed pattern in muricid predation (occurring only in the I.S. 5e). However, the analyzed I.S. 5e mollusks are known to be more time-averaged (deposits in which many generations of individuals are preserved within one stratigraphic layer[32]) than the modern samples[14]. Nonetheless, naticid preferences (e.g. prey type and size) are reliable, long-term responses in time-averaged studies[33]. Among community parameters (e.g. species relative abundance, richness, evenness, habitat, etc.) trophic parameters are less distorted because of time-averaging[17]. Furthermore, abundant species in single live surveys are also dominant in local death assemblages; and abundance data are far less taphonomically ambiguous than species richness[34]. Thus, the comparison between Chione spp. preyed on during I.S. 5e and the modern is appropriate.The 0.7% incompletely drilled and the 9.6% successfully preyed upon Chione spp. are lower than the average 14% of naticid shells drilled successfully, with incomplete drillholes accounting for only 1.4% of the total sample in confamilial individuals from the mid-Atlantic[35]. In a geographically closer location, upper Gulf of California, successful predation by gastropods on Mulinia coloradoensis was 23%[11]. The present results could be underestimates of drilling predation because of possible preferential destruction of drilled valves or because of attacks on shelled prey without drilling[12, 36], or simply because the gastropod predators have many alternative prey (numbers above, 92 and 97 bivalve and gastropod taxa, respectively[14, 30]); on the other hand they can be viewed as overestimates if obliteration of shells by crushing predators was significant[12].The unexpected higher drilling frequency in modern Chione spp. (average 0.13 + 0.02) than in their I.S. 5e counterpart (0.05 + 0.01; also observed and statistically significant using percent preyed upon Chione spp. between I.S. 5e and modern samples: t (0.05) 9,9 = - 4.93, p = 0.001) requires further explanation. With the ~ 2-3℃ warmer temperatures during the I.S. 5e gastropods should have a higher metabolic rate[37] yielding a higher drilling frequency, and thus potentially a smaller population size of Chione spp. However, the higher values observed in today’s samples can be attributed to such temperature differences because shell deposition increases with higher temperatures[38], allowing Chione spp. individuals to increase their shell thickness (this hypothesis will be tested elsewhere) and thus deterring drilling predators more efficiently. If this hypothesis is correct, then one would predict drilling will decrease as the Gulf of California warms, and might even say this could be a positive for the clam’s fishery? Before reaching such conclusion, some alternative hypotheses need to be discussed.One of the apparently strongest (but easy to reject) hypotheses to explain the unexpected higher drilling frequency in modern Chione spp. than in their I.S. 5e counterpart is a higher number of predatory snails in the modern. However, as stated above, this was not the case because the number of I.S. 5e snails was 352 vs. 22 modern ones. Thus, what about differences in predator size[12], being larger in the modern? Also an easily rejected hypothesis because of the lack of differences in drillhole diameters (Fig. 2) between the two times. Alternatively and more feasible, the number of prey were higher in the Pleistocene, as stated above, the number of bivalve taxa found in the studied area was 92 and there were 97 gastropods taxa yielding enough alternative prey for the snails. Furthermore, taxonomic richness was higher in the I.S. 5e than in modern samples[14, 30]. Thus, until the alternative higher number of prey for the Pleistocene snails is not disregarded, the above conclusion of potential fishery’s benefits cannot be supported.Unsurprisingly, Chione spp. individuals are non-randomly drilled by gastropods (mainly naticids: χ2 0.05,8 = 110.45). Stereotypy in drillhole location has been shown in Miocene and recent Naticids[35]. Furthermore, it is suggested that stereotyped behavior increases the probability of successful predation[12], and naticids are known to exhibit stereotypy upon metamorphosis[39], although drillhole position changes ontogenetically (Calvert 1992 in[12]), and even though there are instances of anomalous drillhole placements of naticids[40]; this was not the case in the present study.
ACKNOWLEDGEMENTS
- Constructive criticism on the manuscript by M.S. Foster (Moss Landing Marine Laboratories, MLML) and two anonymous reviewers is acknowledged. The study was possible with the support of the Inter-American Institute for Global Climate Change (Grant UC-AR-S97-74025 to M.S. Foster); National Geographic Society (Grant 5774-96 to K.H. Meldahl, Mira Costa College); Dr. Earl H. Myers and Ethel M. Myers Oceanography and Marine Biology Trust, and Packard Foundation to the author. Field assistance was provided by students from MLML (especially the 1998 Subtidal Ecology course) and from Universidad Autónoma de Baja California Sur under the guidance of R. Riosmena-Rodríguez. This manuscript is dedicated to the memory of my beloved uncle L.F. Cintra Mc Glone.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML