-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(4): 97-102
doi: 10.5923/j.ijmb.20120204.06
Phytochemical Screening of Tacca Leontopetaloides (L.) Kuntze Collected from Four Geographical Locations in Nigeria
T. I. Borokini 1, A. E. Ayodele 2
1National Centre for Genetic Resources and Biotechnology (NACGRAB), Moor Plantation, P. M. B. 5382, Ibadan, Nigeria
2Department of Botany, University of Ibadan, Ibadan, Nigeria
Correspondence to: T. I. Borokini , National Centre for Genetic Resources and Biotechnology (NACGRAB), Moor Plantation, P. M. B. 5382, Ibadan, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study focused on the screening of leaves and tubers of Tacca leontopetaloides (L.) Kuntze collected from different locations for the presence of secondary metabolites and to determine the extent of the environment on these constituents. The study showed the presence of alkaloids, saponins and tannins in the leaf of T. leontopetaloides while only alkaloids were present in the tubers. Variations in the presence of alkaloids and cardiac glycosides in the leaf and tuber samples of the plant were observed. It was noted that environmental factors have a slight effect on the phytochemical content of the same plant in different locations. The pharmacological significance of the secondary metabolites was discussed.
Keywords: Tacca Leontopetaloides, Phytochemical Analysis, Taccaceae, Nigeria, Secondary Metabolites
Cite this paper: T. I. Borokini , A. E. Ayodele , "Phytochemical Screening of Tacca Leontopetaloides (L.) Kuntze Collected from Four Geographical Locations in Nigeria", International Journal of Modern Botany, Vol. 2 No. 4, 2012, pp. 97-102. doi: 10.5923/j.ijmb.20120204.06.
Article Outline
1. Introduction
- Phytochemicals are chemical compounds formed during the plants’ normal metabolically processes[1]. These chemicals are often referred to as secondary metabolites of which there are several classes including alkaloids, flavonoids, coumarins, glycosides, gums, polysaccharides, phenols, tannins, terpenes and terpenoids[2]. According to Heldt[3], most of these phytochemicals are produced through biosynthesis in the metabolic pathways.The knowledge about these phytochemicals, including the discovery of several new organic substances is of importance to many disciplines. These include: Botany (utilization of the chemical and biosynthetic knowledge for studyingsystematics and evolution, as an aid in botanical classification), Ecology (studies on the structural variation of secondary metabolites in space could convey to the discovery of adaptive mechanisms and coevolution of organisms in their ecosystems and to the knowledge of defense, pollination and dispersion strategies of plant species), Pharmacology (chemical diversity of phytochemicals represents an endless source of new drugs and the pharmacological investigation of phytomedicines and accelerate the development of screening techniques), Biotechnology (phytochemical analysis furnishes the background for the selection of species for micropropagation and for monitoring infochemicals produced by cell cultures) among other disciplines[4,5,6,7]. The principles of chemotaxonomy were elaborated in the past century by A. P. De Candolle. He put forward two postulates: (i) Plant taxonomy will be the most useful guide to man in his search for new industrial and medicinal plants; (ii) Chemical characteristics of plants will be most valuable to plant taxonomy in the future[8].Tacca leontopetaloides (L.) Kuntze is a wild perennial herb belonging to the family Taccaceae. Tacca (L.) Kuntze is the only genus in the family Taccaceae, a newly-developed plant family carved out of the Dioscoreaceae, but both families still share a close taxonomic relationship[9]. The plant is native to Malaysia and the Pacific Islands[10,11] and it is naturally distributed from Western Africa, through Southern Asia to northern Australia. Because of its wide distribution, the plant has numerous common and synonymous scientific names, but Polynesian arrowroot appears to be the most widely used. In Yorubaland, Southwest of Nigeria, it is called ‘aduro susu’ or ‘akana maigbo’, while it is called ‘Giginya biri’ or ‘Gaatarin zoomoo’ in Hausa language, Northern Nigeria. The plant is more widespread in the middle belt of Nigeria[12] and in the Southwestern states. This plant produces edible tubers and also fleshy sweet-tasting fruits which are dispersed by birds and mammals[13]. The plant still remains in the wild and is underutilized in Nigeria.In Northern Nigeria, the tubers are eaten especially when other staple foods are[11]. Although the tubers are poisonous, the poison is removed by soaking or washing and rinsing the starchy tubers in water repeatedly, after which they can be processed for food. The tuber contains starch, ceryl alcohol, steroidal saponins and a bitter principle, Taccalin[9]. The grounded root is put on guinea worm infected area to stop the epidemics, and is also taken in infusion to treat hepatitis. In Plateau state of Nigeria, a root preparation is used for snake bite and some other ailments. The water in which the tuber gratings have been washed is used as a detergent[14]. The tubers are a potential starting point for making alcohol; the flowers are rubbed on a snake bite; the fruits are edible; and in Plateau state of Nigeria, the plant finds relevance in traditional worship and sacrifices[14].Furthermore, the bitter raw tubers are used to treat stomach ailments, mainly diarrhea and dysentery in many Polynesian Islands[11,15]. The root starch is used to stiffen fabrics in some of the Islands[14]. In traditional Hawaii medicine, the raw tubers are mixed with water and red clay and consumed to treat diarrhea and dysentery, as well as to stop stomach hemorrhage. In Ivory Coast, a leaf decoction is taken orally for scrotal elephantiasis and for oedema of the stomach[14].Despite the widely achieved importance ofphytochemicals from plants, only very few tropical species have been screened[7]. Therefore, this study was aimed at screening the leaves and tubers of T. leontopetaloides collected from different locations with the aim of discovering its photochemical constituents and to determine the extent to which environmental factors affect the phytochemical contents among the samples.
2. Materials and Methods
2.1. Collection of Plant Materials
- Fresh samples from locations across the country were used for the study (Table 1 and Fig. 1).
|
2.2. Phytochemical Screening
- The leaf and tuber samples of T. leontopetaloides collected from the four different locations were air dried till they were observed to have constant weight. Thereafter, they were pulverized into powdery form, using Thomas Wiley mechanical blender. The powdered leaf samples were screened for the presence of alkaloids, cardiac glycosides, anthraquinones, saponins and tannins. 1. Alkaloids: Drangendoff’s reagent was used and the method described by Harborne[16] was adopted. Grinded and powdered leaves (0.2 g) were extracted with 95% ethanol in a Soxhlet extractor for six hours and the ethanolic extract evaporated to dryness using a vacuum evaporator at 45℃. The residue was redissolved in 5 ml of 1% HCl. The resultant solution was divided into three sections, and to each of these portions, a small quantity of the following was added: (a) 5 drops of Drangendoff’s reagent (Potassium Bismuth Iodide); (b) Mayer’s reagent (Potassiomercuric iodide solution); and (c) Wagner’s reagent (Solution of Iodine in Potassium Iodide). Colour changes were observed to draw inference for each of the three portions.2. Saponins: The persistent frothing test for saponins described by Odebiyi & Sofowora[17] was used. To 1 g of the powdered leaf sample, 30 ml of tap water was added. The mixture was vigorously shaken and heated. The sample was observed for the formation of froth to draw inference.3. Tannins: The method of Trease & Evans[18] was adopted. 0.5 g powdered leaf sample was dissolved in 5 ml of distilled water, then boiled gently and cooled. 1ml of this solution was put in a test tube and 3 drops of ferric chloride solution was added. The colour of the sample was observed to draw inference.4. Cardiac glycosides: The method of Trease & Evans[18] was used. 1 g each of the powdered sample was extracted with 10 ml of 80% alcohol for 5 minutes. The filtrates were diluted with equal volumes of distilled water. Few drops of acetate was added to the diluted filtrate, which turns milky and then filtered. The filtrates were extracted with aliquots of chloroform and then divided into two portions for the Keller-Killani test and Kedde tests. For Keller-Killani test, cooled filtrates from the 4 samples were dissolved separately in 3 ml of FeCl3 reagent (containing 0.3 ml of 10% FeCl3 in 50 ml glacial acetic acid). 2 ml of H2S04 were carefully added to the filtrate and the resulting reaction was observed. For Kedde test, filtrates of the four samples were mixed eith 1 ml of 2% 3,5-dinitobenzoic acid in ethanol. The resulting solutions were made alkaline with 5% NaOH and the colouration was observed to draw inference.5. Anthraquinones: The method described by Trease & Evans[18] was adopted. 5 ml of chloroform was added to 0.5 g of the powdered dry seeds of each specimen. The resulting mixture was shaken for 5 mins after which it was filtered. The filtrate was then shaken with equal volume of 10% ammonia solution. Colour changes were observed to draw inference.
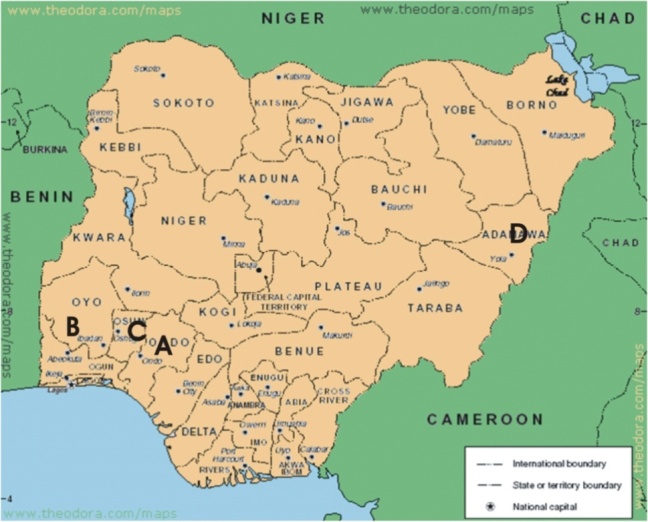 | Figure 1. Map of Nigeria showing the sites of Plant collections |
|
3. Results
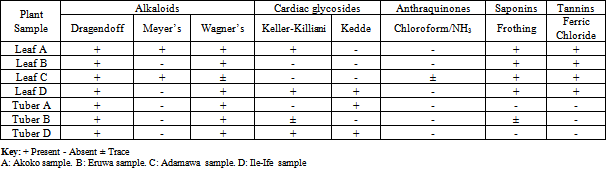
- The results of the phytochemical analysis of the leaf and tuber samples of T. leontopetaloides collected from the four different locations were shown on Table 3. It indicated that using Dragendoff’s test, alkaloids were tested positive in all the leaf and tuber samples; while Meyer’s test indicated the presence of alkaloids only in leaf samples from Akoko (sample A) and Bazza (Sample C). Furthermore, Wagner’s test showed that alkaloids were present in all of the leaf and tuber samples except from leaf sample from Bazza, which was found to be in trace quantities (Table 3).Keller-Killiani reagent gave positive results for cardiac glycosides in leaf samples A (Akoko), D (Ile-Ife) and tuber sample D (Ile-Ife), while it was present in trace in tuber sample B (Eruwa). However, Kedde test gave a slightly different result. Cardiac glycoside was tested present only in leaf sample D (Ile-Ife), tuber samples A (Akoko) and D (Ile-Ife) (Table 3).The Chloroform/Ammonia test showed that Anthraquinones was absent in all the leaf and tuber samples except in leaf sample C (Bazza) where it was found in traces (Table 3). Furthermore, the test for saponins using the frothing test, yielded positive results for all the leaf samples and while tuber sample B (Eruwa) had it in trace, but were observed absent in the remaining tuber samples (Table 3). Tannins, tested using the FeCl3 reagent, indicated that all the leaf samples contains tannins, while it was absent in all the tuber samples (Table 3).
4. Discussions
- The results of this phytochemical screening have shown that environmental factors have a slight effect on the phytochemical content of the same plant in different locations. Variations in the presence of the phytochemicals in the leaf and tuber samples of T. leontopetaloides were observed in the test for alkaloids and cardiac glycosides in the leaf and tuber samples. Using Keller-Killiani test, cardiac glycosides were tested present in leaf sample collected from Akoko (sample A) and Ile-Ife (Sample D), while they were absent in the leaf sample from Eruwa (sample B) and Bazza (sample C). However, the test for the remaining secondary metabolites showed very little variations among the leaf and tuber samples. However, environmental factors have been identified as responsible for changes and determination of the secondary metabolites in a plant[19]. As such, same plant from different environments could have different phytochemical content. Previously, Randrianalijaona et al [20] reported the seasonal changes in the chemical composition of essential oils in more than seventy L. camara from different parts of the world. Similarly, Bhakta & Ganjewala[21] reported ontogenic variation in secondary metabolites such as phenolics, anthocyanins, and proanthocyanidins in L. camara. In addition, Fonseca et al[22] confirmed the fluctuation of secondary metabolite contents in medicinal plants with changing environment. In addition, Odabas et al[23] reported increases in the content of hyperforin, hypericin and pseudohypericin with increase in temperature and light in Hypericum perforatum. Furthermore, Lester & Eischen[24] reported variations on the beta-carotene content of muskmelon grown on different soil conditions; while water availability in the soil was reported to have effect on the carotenoid level in grapes[25]. Furthermore, Wang et al[26] reported different levels of flavonoids, anthocyanins and phenolic acids in strawberries under different soil treatments. Several other authors also reported how different climatic conditions could affect the phytochemical contents of plants[27,28]. These and many more previous studies indicate that environmental factors could be responsible for variations in the production of secondary metabolites in plants. Several other factors, apart from environmental factors, that can affect the phytochemical contents of plants include the use of chemicals (pesticides), genetic factors, diseases and pests, fertilization, the period of harvesting and many others[29]. Furthermore, discrepancies were observed in the results of the phytochemical screening of the leaf and tuber samples of plant studied, with respect to the test for alkaloids and cardiac glycosides using different reagents. Similar false positive results were obtained for tannins, flavonoids, cardiac glycosides and alkaloids in a phytochemical screening of some selected medicinal plants in Northern Thailand[30]. It is very common to encounter false positive results in phytochemical screening, that’s why the use of more than one reagent in testing for secondary metabolites is recommended for confirmation and reliability. This was corroborated by Segelman et al[31].This study has confirmed the presence of alkaloids, saponins and tannins in T. leontopetaloides leaf while only alkaloids were present also in the tubers. Alkaloids, comprising a large group of nitrogenous compounds are widely used as therapeutic agents in the management of cancer[32]. Tannins are also well known for their anti-microbial properties; they have soothing relief, helps to regenerate the skin, it is anti-inflammatory and diuretic[33]. Tannins are complex phenolic polymers which can bind to proteins and carbohydrates resulting in reduction in digestibility of these macromolecules and thus inhibition of microbial growth[34]. Tannins are reported to have astringent properties on mucous membranes[35]. In addition to all these, tannins are also used in the treatment of wounds emanating from varicose ulcers andhaemorrhoids[36] and to stop bleeding during circumcision[37]. Saponins are glycosides of both triterpenes and steroids having hypotensive and cardiac depressant properties, and have been detected in over seventy plant families[38,39]. They have been shown to possess beneficial properties by lowering the cholesterol level, have anti-diabetic and anticarcinogenic properties[18] as well as being used as an expectorant and emulsifying agent[40]. Saponins are reported as a major component acting as antifungal secondary metabolite[41].These results have shown the presence of important secondary metabolites in T. leontopetaloides that have significance in ethnomedicine and ethnopharmacology. Furthermore, it is believed that these results will prove useful in the assessment of environmental effects on the synthesis of phytochemicals at species’ level.
References
| [1] | Okigbo R.N. Anuagasi C.L. and Amadi J.E. “Advances in selected medicinal and aromatic plants indigenous to Africa” Journal of Medicinal Plants Research 3(2), pp 86-95, 2009. |
| [2] | Okwu D.E. “Phytochemicals and vitamin content of indigenous spices of South Eastern Nigeria”, Journal of Sustainable Agriculture and Environment 6, pp 30-34, 2004. |
| [3] | Heldt H. Plant Biochemistry, 1st ed., Academic Press/Elsevier, New Delhi, India, 2005. |
| [4] | Marjorie M.C. “Plant Products as Antimicrobial Agent”, Clinical and Microbiology Reviews 12 (4), pp 564-582, 1999. |
| [5] | Raimundo B.F. “Brazilian Phytochemical Diversity: Bioorganic Compounds Produced by Secondary Metabolism as a Source of New Scientific Development, Varied Industrial Applications and to enhance Human Heath and the Quality of Life”, Journal of Pure and Applied Chemistry 71(9), 663-1672, 1999. |
| [6] | Akenga T. Orech F.O. Ochora J. Friis H. and Aagaard H. “Potential Toxicity of some Traditional Leafy Vegetables consumed in Nyang’oma Division, Western Kenya”, African Journal of Food and Nutritional Science 5 (1), pp 1-30, 2005. |
| [7] | Edeoga H.O. Okwu D.E. and Mbaebie B.O. “Phytochemical Constituents of some Nigeria Medicinal Plants”, African Journal of Biotechnology 4 (7), pp 685-688, 2005. |
| [8] | De Candolle A. P. Essai sur les propridtds médicales des plantes, cornparees avec leurs forms extérieures et leur classification naturelle. (Second edition), Paris, France, 1816. |
| [9] | Caddick R.L. Wilkin P. Rudall P.J. Hedderson A.J. and Chase M.W. “Yams reclassified: A recircumscription of Dioscoreaceae and Dioscoreales” Taxon 51, pp 103 – 114, 2002. |
| [10] | Purseglove J.W. Taccaceae. Tropical Crops. Monocotyledons 2, 1st Ed., Longman Group Ltd, London, United Kingdom, 1972. |
| [11] | Kay D.E. Root Crops, 2nd Ed., In: Gooding E.G.B (ed.), Tropical Development and Research Institute, UK (now, National Resources Institute, UK), 1987. |
| [12] | Manek R.V. Kunle O.O. Emeje M.O. Builders P. Rama Rao G.V. Lopez G.P. and Kolling W.M. “Physical, Thermal and Sorption profile of starch obtained from Tacca leontopetaloides”, Starch 57, pp 55 – 61, 2005. |
| [13] | Drenth E. “A revision of the family Taccaceae”, Blumea 20, pp 367 – 406, 1972. |
| [14] | Ukpabi U.J. Cassava processing and Utilization, A Sensitization book, 1st Ed., NRCRI, Umuahia, Nigeria, 2009. |
| [15] | Brand-Miller J. James K.W. and Maggiore P. Tables of composition of Australian Aboriginal Foods, 1st Ed. Aboriginal Studies Press, Canberra, Australia, 1993. |
| [16] | Harborne J.B. Phytochemical methods, 3rd ed., Chapman and Hall Ltd, London, 1973. |
| [17] | Odebiyi O.O. and Sofowora E.A. “Phytochemical screening of Nigerian medicinal plants”, Lloydia, 41(3), pp 234-246, 1978. |
| [18] | Trease G.E. and Evans W.C. Pharmacognosy. Thirteenth Ed., Balliere Tindall, London, United Kingdom, 1989. |
| [19] | Waterman P.G. and Mole S. Entrinsic factors influencing production of secondary metabolites in plants. In: Bernays EA (Ed) Insect – plant interactions, Volume 1, CRC Press, Boca Ranton, FL, USA, 1989. |
| [20] | Randrianalijaona J.A. Ramanoelina P.A.R. Rasoarahona J.R.E. and Gaydouet E.M. “Seasonal and chemotype influences on the chemical composition of Lantana camara L.: Essential oils from Madagascar”, Analytica Clinica Acta 545, pp 46-52, 2005. |
| [21] | Bhakta D. and Ganjewala, D. “Effect of leaf positions on total phenolics, flavonoids and proanthocyanidins content and antioxidant activities in Lantana camara (L)”, Journal of Science Research 1, pp 363-369, 2009. |
| [22] | Fonseca J.M. Rushing J.W. Rajapaske N.C. Thomas R.L. and Riley M.B. “Potential implications of Medicinal Plant Production in Controlled Environments: The case of Feverfew (Tannacetum parthenium)” HortScience 41 (3), pp 531 – 535, 2006. |
| [23] | Odabas M.S. Raduienë J. Camas N. Janulis V. Ivanauskas L. and Cirak C. “The quantitative effects of temperature and light intensity on hyperforin and hypericins accumulation in Hypericum perforatum L.” Journal of Medicinal Plants Research 3(7), pp 519-525, 2009. |
| [24] | Lester G.E. and Eischen F. “Beta-carotene content of postharvest orange-fleshed muskmelon fruit: Effects of cultivar, growing locations and fruit size”, Plant Foods and Human Nutrition 49, pp 191 – 197, 1996. |
| [25] | Oliveira C. Silva Ferreira A.C. Mendes Pinto M. Hogg T. Alves F. and Guedes de Pinho P. “Carotenoid compounds in grapes and their relationship to plant water status”, Journal of Agriculture and Food Chemistry 51, pp 5967 – 5971, 2003. |
| [26] | Wang S.Y. Zheng W. and Galleta G.J. “Cultural system affects fruit quality and antioxidant capacity in strawberries”, Journal of Agriculture and Food Chemistry, 50, pp 6534 – 6542, 2002. |
| [27] | Howard L.R. Pandjaitan N. Morelock T. and Gil M.I. “Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season”, Journal of Agriculture and Food Chemistry 50, pp 5891 – 5896, 2002. |
| [28] | Vallejo F, Tomas-Barberan F.A. Gonzalez Benavente-Garcia A. and Garcia-Viguera C. “Total and individual glucosinolate contents in inflorescences of eight broccoli cultivars grown under various climatic and fertilization conditions”, Journal of Science, Food and Agronomy 83, pp 307 – 313, 2003. |
| [29] | Zhao X. Carey E.E. Wang W. and Rajashekar C.B. “Does organic production enhance phytochemical content of fruit and vegetables? Current knowledge and prospects for research”, HortTechnology 13 (3), pp 449 – 456, 2006. |
| [30] | Panyaphu K. Sirisa-ard P. Na Ubol P. Nathakarnkitkul S. Van On T. “Phytochemical, antioxidant and antibacterial activities of medicinal plants used in Northern Thailand as postpartum herbal bath recipes by the Mien (Yao) community”, Phytopharmacology 2(1), pp 92-105, 2012. |
| [31] | Segelman A.B. Farnsworth N.R. and Quimby M.W. “Biological and phytochemical evaluation of plants, III: False-negative saponin test results induced by the presence of tannins”, Lloydia 32, pp 52-58, 1968. |
| [32] | Chabner B.A. and Howitz T.L. “Plant Alkaloids”, In: Pinedo HM, Chabner BA, Longo DL (eds.). Cancer Chemotherapy Biological Responses 66, pp 627- 637, 1990. |
| [33] | Okwu D.E. and Okwu M.E. “Chemical composition of Spondias mombin Linn Plant parts”, Journal of Sustainable Agriculture and Environment 6(2), pp 140 – 147, 2004. |
| [34] | Nwogu L.A. Igwe C.U. and Emejulu A.A. “Effects of Landolphia owariensis leaf extract on the liver function profile and haemoglobin concentration of albino rats”, African Journal of Biotechnology 2(12), pp 240-242, 2008. |
| [35] | Egunyomi A. Moody J.O. and Eletu, O.M. “Antisickling activities of two ethnomedicinal plant recipes used for the management of sickle cell anaemia in Ibadan, Nigeria”, African Journal of Biotechnology 8(1), pp 020-025, 2009. |
| [36] | Nguyi A.A. “Tannins of some Nigerian flora”, Nigerian Journal of Biotechnology 6, pp 221-226, 1988. |
| [37] | Joshua K. “Conservation of Indigenous Medicinal botanicals in Ekiti State, Nigeria”, Journal of Zhengheng University Science B 7, pp 713- 718, 2006. |
| [38] | Basu N. and Rastogi R.P. “Triterpenoid, Saponins and Sapogenins”, Phytochemistry 6, pp 1249-1270, 1967. |
| [39] | Olaleye M.T. “Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa”, Journal of Medicinal Plants Research 1(1), pp 009-013, 2007. |
| [40] | Edeoga H.O. Omosun G. and Uche L.C. “Chemical composition of Hyptis suaveolens and Ocimum gratissimum hybrids from Nigeria”, African Journal of Biotechnology 5 (10), pp 892-895, 2006. |
| [41] | Onwuliri F.E. and Wonang D.L. “Biochemical and antimicrobial studies of Zingiber officinale and Allium sativum on the selected microorganisms”, University of Jos, Nigeria, 2003. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML