-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(4): 83-87
doi: 10.5923/j.ijmb.20120204.03
Changes in Antioxidative Responses to Low Temperature in Tea[Camellia sinensis (L) O. Kuntze] Cultivars
Hrishikesh Upadhyaya
Department of Botany and Biotechnology, Karimganj College, Karimganj, 788710, Assam, India
Correspondence to: Hrishikesh Upadhyaya , Department of Botany and Biotechnology, Karimganj College, Karimganj, 788710, Assam, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Effect of low temperature stress treatment in five selected clones of Camellia sinensis (T-17, TV-19, TV-25, TV-29, TV-30) was found to be significant. Guaiacol Peroxidase (GPX) decreased with decreasing temperature. PPO decreased with decreasing temperature showing highest activities at 20℃ in all clones. CAT activities decreased in T-17, TV- 29, and TV-30 with decrease in temperature with exceptions in TV-19, and TV- 25 which showed increase catalase ( CAT) and guaiacol peroxidase (GPX) activities. A decreasing trend of lipid peroxidation was observed in all the clones with decreasing temperature. Total phenolics content was highest in all clones at 20℃ with exception in TV- 30, which showed lowest at 20℃. Total sugar content was found to be highest in all clones treated at 20℃. The present study indicates that various clones of tea showed a range of physiological and biochemical sensitivities under a low temperature stress
Keywords: Camellia Sinensis, Antioxidative Responses, Low Temperature
Cite this paper: Hrishikesh Upadhyaya , "Changes in Antioxidative Responses to Low Temperature in Tea[Camellia sinensis (L) O. Kuntze] Cultivars", International Journal of Modern Botany, Vol. 2 No. 4, 2012, pp. 83-87. doi: 10.5923/j.ijmb.20120204.03.
Article Outline
1. Introduction
- Tea is the most economic plant as it is the one of important beverages of the world. Tea is produced from the leaves of Camellia sinensis. In India tea plants are cultivated in the Barak and Brahmaputra Valley in Assam and in the hilly terrains of Darjeeling and other parts of the country. Being perennial crop, tea is subjected to different biotic and abiotic stresses. Abiotic stress includes temperature, drought, metal stress etc. Such stresses in plants are marked by the production of reactive oxygen species (ROS) like, hydroxyl radical (OH), superoxide radical (O2), alkoxyl radical (RO) hydroperoxide radical (HO2), hydrogen peroxide (H2O2) etc[1-2]. These reactive oxygen species (ROS) are the products of natural redox reactions occurring in various cellular compartments and degrade the important macro molecules like nucleic acid, proteins, lipids and also caused pigment photobleaching ([3-5]. But to adapt themselves to various stresses, plant develops both enzymic (Catalase, Guaiocol peroxidase, Glutathione reductase, Superoxide dismutase, Ascorbate peroxidase) and non- enzymic (carotenoid, glutathione, ascorbate α -tocopherol) antioxidant protection to overcome the cytotoxic effect of ROS[6-8]. Temperature is one of the important factor, which affects the metabolism in plants. Tea plant in particular grows in shade and being C3 plant is temperature sensitive. The capacity of plants to adapt themselves to different temperatures can be attributed to changes in the key components in cellular constituents enabling plants to work efficiently under various temperature regimes[9]. The variation of photosynthetic rates in different clones of tea in response to different temperature regime is well documented[10,11].Polyphenols being the major constituents of tea leaves, plays important role in plants defense metabolism[12]. Total polyphenolics content in tea includes catechins, epicatechins etc. Polyphenols present in tea are the good source of antioxidants in plants[13]. Variations in antioxidant property among the various clones of Camellia sinensis has also been reported[2,14]. Polyphenol oxidase, (PPO) is one of the major enzyme involved in phenol metabolism. PPO catalyzes the oxidation of O-diphenols to their corresponding quinones, which are then sponeneously transformed to more complex fermentation products – a key step in tea processing[15]. Thus the involvement of PPO in producing characteristics flavour of tea leaves has also been reported[16]. High temperature induced changes phenol metabolism in tea leaves has also been reported by[11]. The present investigation was undertaken to determine the biochemical response to low temperature in five clones of tea plants.
2. Materials and Methods
2.1. Plant Material
- Five clonal varieties of healthy and uniform seedlings of tea[Camellia sinensis (L)O Kuntze] of one year old were procured from Tocklai Tea Research Station, Silchar and were acclimatized in laboratory conditions. The five clones (TV – 19, TV – 25, TV – 29, TV – 30 and T – 17) of tea plants were kept in temperature regulated chamber for four hours under continuous light. The plants were kept at 10℃, 20℃ and 30℃ for four hours each and after each treatment tea leaves were sampled for biochemical and enzymic estimations.
2.2. Lipid Peroxidation , Total Sugar and Phenolics Content
- Lipid peroxidation was measured as the amount of TBARS chiefly MDA determined by the thiobarbituric acid (TBA) reaction as described by Heath and Packer[17]. The leaf tissues (0.2 g) were homogenized in 2.0 ml of 0.1 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000g for 20min. To 1.0 ml of the resulting supernatant, 1.0 ml of TCA (20%) containing 10.5% (w/v) of TBA and 10.0 cm6 (4.0 % in ethanol) BHT (butylated hydroxytolune) were added. The mixture was heated at 95℃ for 30 min in a water bath and then cooled in ice. The contents were centrifuged at 10,000 g for 15 min and the absorbance was measured at 532 nm and corrected for 600 nm. The concentration of MDA was calculated using extinction coefficient of 155m M-1cm-1. Total phenolics were extracted from the tea leaves as per the method of Harbone[18] and estimated following the method of Mahadevan and Sridhar[19], using Folin-Ciocalteu reagent and Na2CO3. Aliquots from the 80% ethanol extract was taken for the estimation of the total soluble sugar by Anthrone’s reagent[20]
2.3. Extraction and Assay of Enzymes
- The leaves tissues were homogenised with phosphate buffer pH (6.8) (0.1M) in prechilled mortar and pestle. The extract was centrifuged at 4℃ for 15 min at 17,000g in a cooling centrifuge. The supernatant was used for assay of guaiacol peroxidase (GPX), catalase (CAT) and polyphenol oxidase (PPO). The GPX and CAT activities were assayed as per the method of Chance and Maehly[21]. The assay of SOD was done as per the method of Giannopolitis and Reis[22]. Assay of polyphenol oxidase (PPO) was done as per the method of Kar and Mishra[23].
2.4. Statistical Analysis
- All the experiments were repeated three times and the data presented are mean ± SE. Data analysis were done by using SPSS 10 for multiple comparision between treatment by Tukey test at P<.05.
3. Results and Discussion
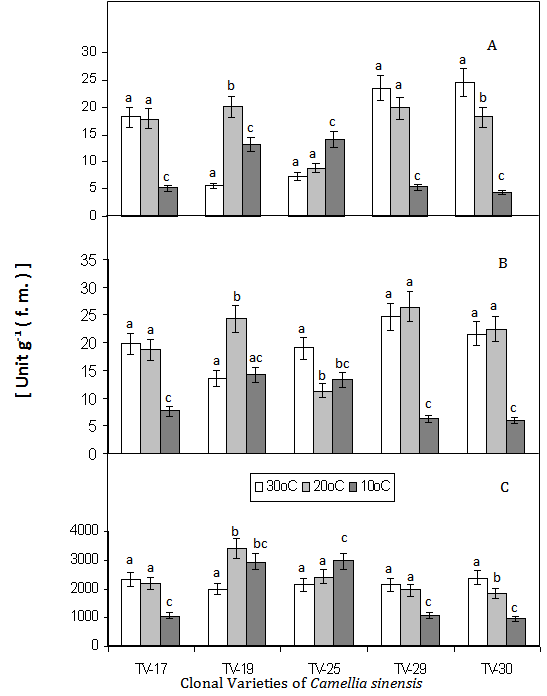
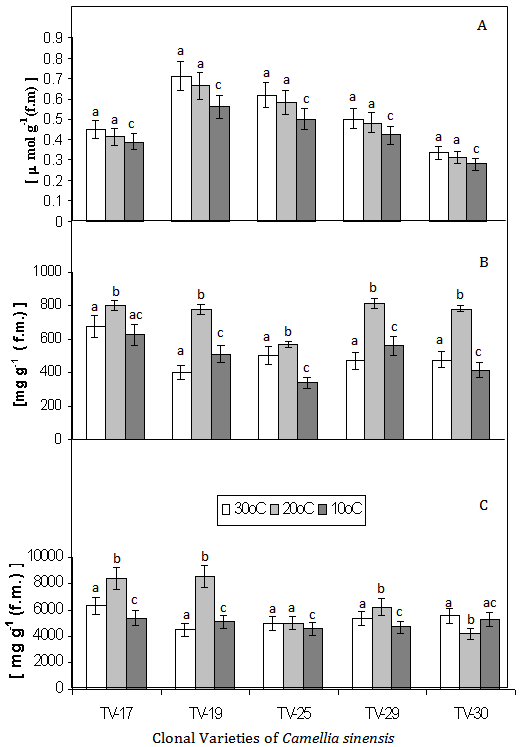
- The effect of low temperature in the selected five clones of Camellia sinensis was found to be significant. The guaiacol peroxidase (GPX) was found to be decreased with decreasing temperature (30 , 20 and 10℃), with highest activity in TV-30, at 30℃, which was taken as control, but TV–25, showed increased activity with the decrease in temperature (Fig. 1 A). GPX has been suggested to be involved in scavenging of H2O2, under stress conditions[24, 1, 2]. The low GPX activity levels and the lack of inducibility of the enzyme during the low temperature condition of tea seedlings would suggest that, GPX is not the main H2O2 scavenger in four (T–17, TV–19, TV–29 and TV–30) clones under low temperature condition, as was observed by others in jackpine[25]. But increase GPX activity during similar condition in TV–25 can be attributed to the better resistance and adaptability of the variety to low temperature stress. Polyphenol peroxidase (PPO) was found to be decreased with decreasing temperature in T–17 and TV–25 with highest activity shown by TV–29 and TV–30. In all the varieties PPO activity is highest at 20℃, which are 0.94, 0.176, 0.59, 1.06 and 1.04 fold increase in T–17, TV–19, TV–25, TV–29 and TV–30 respectively (Fig. 1-B). Polyphenol oxidase (PPO) are widely distributed in the plant and play a role in oxygen scavenging and defense against stress. PPO activity was found to be highest in all variety at 20℃. PPO plays important role in phenol metabolism. PPO catalyses the O2-dependent oxidation of mono- and odiphenols to o-diquinones, where secondary reactions may be responsible for the defense reaction and hypersensitive response[26]. Moreover, it is proposed that PPO activity may regulate the redox state of phenolic compounds and become involved in the phenylpropanoid pathway. The catalase (CAT) activities decreases with decrease in temperature in T–17, TV–29 and TV-30, being highest activity at 30℃ in TV–30, but a increase CAT activity was observed in TV–19 and TV–25 with decrease in temperature. The increase in CAT at 20℃ and 10℃ in TV – 19 and TV – 25 was found to be 1.71 and 1.47 and 1.13 and 1.38 fold respectively (Fig.1C). Declining trend of CAT activity with decreasing temperature in three varieties (T-17, TV–29 and TV – 30) suggests the accumulation of free radicals and causes greater damage to cell at low temperature. But TV–19 and TV–25 showed increase CAT activity at low temperature, suggesting the less accumulation of toxic H2O2 and thus greater adaptability to low temperature stress.Decrease in lipid peroxidation with decreasing temperature was observed in all the tested cultivars. However, lipid peroxidation was highest among TV-19 and TV - 25 and lowest in TV-17 and TV-30 as depicted in Fig. 2 A. This indicates that in comparision to other clones, TV-17 and TV-30 showed more membrane stability which could be attributed to their better antioxidant efficiency which was already reported[2,7,8,14].Phenolic compounds are widely distributed in plants and are mainly produced to protect plants from stress, ROS, wounds, UV light, disease and herbivores. Tea polyphenols are mostly catechins which have potential antioxidant properties that makes tea a good health drink[13,15]. Total phenolic content in low temperature treated clones, T-17, TV-19, TV-25, TV-29 was found to be highest at 20℃, but TV-30 showed low content of the same at 20℃, which is only 0.75 fold and highest content at 30℃ (Fig. 2C). This suggested that TV-30 is low temperature sensitive, from phenol metabolism point of view. High temperature induced changes in phenol metabolism in tea leaves was already reported[11]. However, phenolic content observed in other four varieties (T–17, TV–25, TV–29, TV–19) showed, a relatively low temperature resistance in such variety. Total sugar content was found to be highest in all varieties treated with 20℃ which are, 1.98, 3.17, 1.13, 1.73 and 1.63 fold increase in T–17, TV–19, TV–25, TV–29 and TV–30 respectively (Fig. 2-B). Higher total sugar content in all clones treated at 20℃ suggested the better photosynthetic activity in all these clones at such temperature.
4. Conclusions
- Tea is an important economic crop growing in different regions in India. Tea being perennial in nature encounters various environmental conditions which includes the low temperature stress. From the present study, it can be concluded that various clones of tea showed a range of physiological and biochemical sensitivities under a low temperature stress, which could be attributed to variation of their antioxidative responses under low temperature stress.
ACKNOWLEDGEMENTS
- The authors thank , General Manager, Tocklai Tea Estate, Silcoorie, Silchar for providing tea seedlings throughout the experimental work done at Assam University, Silchar.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML