-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
2012; 2(3): 35-39
doi: 10.5923/j.ijmb.20120203.01
Tribulus Terrestris L. (Zygophyllaceae) Flavonoid Compounds
Noori Mitra 1, Dehshiri Mohammad-Mehdi 2, Zolfaghari Mohammad Reza 3
1Department of Biology, Faculty of Science, Arak University, Arak 38156-8-8349-Iran
2Department of Biology, Boroujerd Branch, Islamic Azad University, Boroujerd-Iran
3MSc student of Department of Biology, Boroujerd Branch, Islamic Azad University, Boroujerd-Iran
Correspondence to: Noori Mitra , Department of Biology, Faculty of Science, Arak University, Arak 38156-8-8349-Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study, root, leaf and fruit flavonoids of Tribulus terrestris L. are compared. Aqueous-ethanolic extracts of collected plant material were examined to practice flavonoid detection, isolation and identification by 2-dimensional paper chromatography, thin layer chromatography and available references. Voucher specimen was prepared for reference as herbarium voucher. Results showed chrysin was just identified in fruit while root and leaf had not any chrysin. Also flavone C and C-/O-glycosides were not found in leaf and fruit whereas root of the species had flavonoid sulphates and aglycones in addition to flavone C and C-/O-glycosides.
Keywords: Tribulus Terrestris L., Zygophyllaceae, Flavonoid Compounds, Chromatography
Article Outline
1. Introduction
- The genus Tribulus L. from Zygophyllaceae family and section Terrestris L. consists of 25 species in the world[1-4]. Rechinger has reported four species in Iran that Tribulus terrestris is one of them. It is an annual herb of worldwide distribution that is characterized by having spiny mericarps of fruit[5]. It can thrive even in desert climates and poor soils[6]. The species is used in the folk medicine in India, China, Bulgaria and other countries against various diseases such as cardiac, edema, eye trouble, skin disorders, urinary troubles and stones in the bladder, as a diuretic, aphrodisiac. Recently, anti-tumoural activity and effects on cardiovascular system have been also found[7, 8, 9, 10]. The species is reported to contain steroidal saponins, alkaloids and flavonoids[8]. As Gomathi et al (2012) showed that the aqueous and ethanolic extracts of Tribulus terrestris contain alkaloids, tannin, flavonoids, quinines and phenolic compounds. They found that the aqueous extract of Tribulus terrestris possessed greater antibacterial activity than ethanolic extract and inhibited the growth of selected bacteria and fungi indicating broad spectrum bioactive nature of Tribulus terrestris[11,12]. The activity of the plant against both Gram-positive and Gram-negative bacteria may be indicative to the presence of broad spectrum antibiotic compounds or simply general metabolic toxins in the plant, in addition to the plant (fruits, leaves and root) content of pharmacological active metabolites like furostanol and spi- rostanolsaponins[13], flavonoid glycosides[14], phytosterols and some amides[10]. Wu et al (1999) found that the quantity of main flavonoids is about 1.5 times that of main saponins. This indicated that the flavonoids contents in T. terrestris should be studied, developed, and further used[8]. Louveaux et al (1998) detected eighteen flavonoids (caffeoyl derivatives, quercetin glycosides, including rutin, and kaempferol glycosides) using high-performance liquid chromatography in four Tribulus species leaf extracts. Their data showed that the desert locust prefers T. terrestris plants rich in quercetin glycosides[15]. Also, two known flavonoid glycosides were isolated from the fruits of Tribulus terrestris[16]. Yang et al (2010) worked on Tribulus terrestris extraction of whole plant flavonoid for finding optimum extraction condition using orthogonal experiment. Their results showed the best extraction conditions are as follows: time 1.5h, temperature 70-75℃, ethanol 70% and solid to liquid ration of 1: 15 (g/ml)[17]. Qin et al (1999) found that the flavonoids content of Tribulus terrestris are mainly quercetin, kaempferol and isorhamnetin, and quercetin as a nucleus the highest content of flavonoids[18]. Also Temraz et al. (2006) reported six flavonol glycosides based on kaempferol, isorhamnetin and quercetin aglycone from Tribulus alatus growing in Egypt[19]. Matin Yekta et al (2008) isolated and characterized three flavonoid glycoside from aerial parts of collected Tribulus terrestris L. var. orientalis (Kerner) G. Beck in northeast of Iran. These flavonoids were quercetin 3-O-glycoside, quercetin 3-O-rutinoside and kaempferol 3-O-glycoside[20]. Raja and Venkataraman (2011) showed that the petrolium ether and chloroform extract of Tribulus terrestris fresh fruit from India contains flavonoids. In all the fruit extracts of Tribulus alatus did not find the flavonoids. But thin layer chromatography of the benzene fruit extract shows maximum spots in ethyl acetate: benzene (1:9) solvent system. It shows that all the pharmacognostical characters can be used as a diagnostic tool for the correct identification of the drug and also to test adulteration if any[21]. Therefore, depth study of Tribulus L. medicinal ingredients and flavonoids can provided the basis for further development and utilization. Also flavonoids as secondary metabolites are valuable and widely and effectively used in chemosystematics[22]. They occur widely in plant organs and are a biologically major and chemically diverse group of secondary metabolites that are popular compounds for chemotaxonomic surveys of plant genera and families[23]. Plant phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level[23, 24, 25]. In this study, root, leaf and fruit flavonoids of Tribulus terrestris L. species aqueous-ethanolic extracts are reported.
2. Materials and Methods
2.1. Collection of Plant Material and Preparation
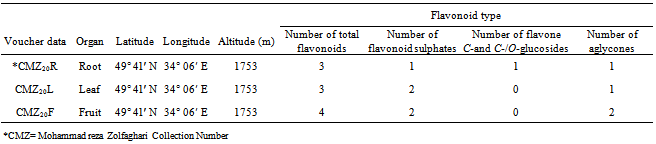
- Mature fresh root, leaf and fruit of Tribulus terrestris species were collected from Markazi Province, Iran area during 2011 as described in Table 1. Plant identified using available references[26-28]. Voucher specimens of each sample were prepared for reference as herbarium vouchers. Samples were air dried for detection and identification of their flavonoids.
2.2. Extraction of the Plant Material
- For a comparative analysis of the flavonoids, small extracts of all the accessions were prepared by boiling 200 mg of powdered air dried root, leaf and fruit for 2 min in 5 ml of 70% EtOH. The mixture was cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40℃, and taken up in 2 ml of 80% MeOH for analysis by 2-Dimensional Paper Chromatography (2-D PC).
2.3. Flavonoid analysis by 2-Dimensional Paper Chromatography (2-D PC)
- For the detection of flavonoids, ca 20 μl of each of the small extracts was applied to the corner of a quarter sheet of Whatman No 1 chromatography paper as a concentrated spot (10 applications of 2μl). The chromatogram for each sample was developed in BAW (n-BuOH-HOAc-H2O=4:1:5; V/V; upper layer), 1st direction, and HOAc (=15% aqueous acetic acid), 2nd direction, with rutin (= quercetin 3-O-rutinoside) as a standard. After development, the chromatograms were viewed in long wave UV light (366 nm) and any dark absorbing and fluorescent spots were marked. Rf -values in BAW and 15% HOAc were calculated.
2.4. Methods of Identification of the Flavonoids
- After obtaining sufficient amounts of purified flavonoids, as in the case of the flavonoids from root, leaf and fruit of the species, they were identified by means of UV spectroscopy using shift reagents to investigate the substitution patterns of the flavonoids[29, 30] and by acid hydrolysis to identify the aglycone and sugar moieties. Cochromatography with standards was also performed where possible. Flavonoid standards available for comparison during the study were Apigenin, Chrysin, Isorhamnetin, Kaempferol, Luteolin, Morine, Myricetin, Naringenin, Quercetin, Rhamnetin, Rutin, Tricine and Vitexin (all obtained commercially, Rutin from Merck, Apigenin and Luteolin from Sigma and the rest from Fluka).
2.5. Acid Hydrolysis and Identification of Flavonoid Aglycones
- A small amount of each purified flavonoid (ca 0.5 mg) was dissolved in 0.5 ml of 80% MeOH in a test tube. To this sample 2 ml of 2M HCl was added and the mixture was heated in a water bath at 100℃ for 0.5 h. The solution was cooled, 2 ml of EtOAc were added and thoroughly mixed with the aqueous layer using a whirley mixer. The upper EtOAc layer was removed with a pipette, evaporated to dryness, dissolved in 0.5 ml of MeOH and applied as spots on thin layer chromatograms (cellulose). The TLC plates were run in three solvents alongside standards to identify the aglycone moiety[31].
3. Results
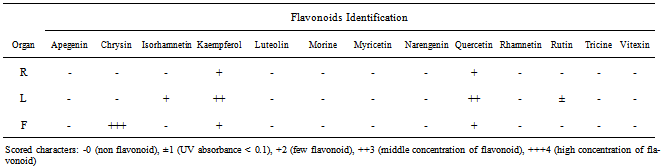
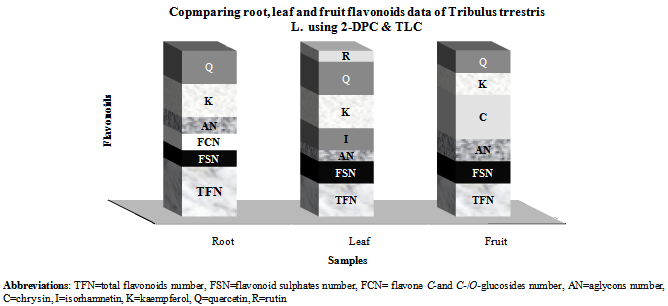
- Results showed root, leaf and fruit of Tribulus terrestris species contained flavonoid compounds. Data in Tables 1 and 2 show the collection information, 2-Dimentional Paper and thin layer chromatographical data of root, leaf and fruit of the species. Figure 1 shows stacked column with a 3-D visual effect histogram for comparing root, leaf and fruit flavonoids data (number of total flavonoids, number of flavonoid sulphate, number of flavone C-and C-/O-glucosides, number of aglycones and occurrence and concentrations of chrysin, isorhamnetin, kaempferol, quercetin and rutin in the species. As Table 1 and 2 and Figure 1 show root, leaf and fruit of the species contain flavonoid sulphates and aglycones whereas flavone C and C-/O-glycosides were found in root but leaf and fruit lack. Root, leaf and fruit contained quercetin and kaempfrol, but isorhamnetin and rutin found in leaf. Also chrysin was just identified in fruit while root and leaf had not any Chrysin.
4. Discussion and Conclusions
- As Table 1 and Figure 1 show there are flavonoid compounds in Tribulus terrestris root, leaf and fruit extracts, but flavone C-and C-/O-glucoside was not observed in leaf and fruit. Saleh et al (1982) studies showed existing flavonoid glycosides in whole plant[14]. Also Wu et al (1999) reported flavonoid content in the species[8]. Gomathi et al (2012) showed existing flavonoid compounds in ethanolic extract of T. terrestris[11]. Chemical study of T. terrestris root, leaf and fruit using thin layer chromatography (TLC) showed quercetin and kaempferol are the most representative compounds for the species that found in all three organs. But, isorhamnetin and rutin found in leaf. Also Chrysin was just identified in fruit while root and leaf had not any Chrysin (Table 2 and Figure 1). Louveaux et al (1998) detected eighteen flavonoids (caffeoyl derivatives, quercetin glycosides, including Rutin, and Kaempferol glycosides) using high-performance liquid chromatography in four Tribulus species leaf extracts[15]. Qin et al (1999) found that the flavonoids content of T. terrestris are mainly Quercetin, kaempferol and isorhamnetin, and quercetin as a nucleus the highest content of flavonoids[18]. Also Temraz et al. (2006) reported six flavonol glycosides based on kaempferol, isorhamnetin and quercetin aglycone from T. alatus growing in Egypt[19]. Matin Yekta et al (2008) isolated and characterized Quercetin 3-O-glycoside, quercetin 3-O-rutinoside and kaempferol 3-O-glycoside from aerial parts of collected T. terrestris L. var. orientalis (Kerner) G. Beck in northeast of Iran[20]. Studying flavonoid pattern can be used for chemosystematic and lower taxonomic levels. As Harborne (1971) studies showed flavonoids may be useful taxonomic markers within the Cyperaceae family[32]. Twelve species of the genus Ephedra have been surveyed[33]. Twenty five Avena species (Poaceae) were investigated for the flavonoid content of leaf tissue[34]. Studies of seventeen Euphorbia species leaf flavonoids showed some phytochemical characters such as total number of flavonoids, kaempherol, quercetin, myricetin, flavone and dihydroflavonol glycoside are valuable for their chemotaxonomy and usage[25]. Diploid triticum species could be divided into two groups depending on the presence or absence of two major di-C-glycosyl flavones[35]. Above mentions show that flavonoids are popular for chemosystematic studies because, they are often reasonably easy to identify, they often show correlations with existing classifications at the taxa level and support revisions of existing classifications. This study shows flavonoids in different organs of T. Terrestris. But further work is needed about another species of the genus Tribulus and Zygophyllaceae family.Finally T. terrestris is weed and grow in poor soils, desert and destroyed pasture. Progress continues to be made in understanding the roles of flavonoids in stress protection, as well as in defining the mechanisms that control the amount and varieties of flavonoids that are produced in plants in response to diverse environmental cuse[36]. In addition T. terrestris is used in folk medicine against various diseases and some of its theraputic features have been identified[37]. Also the quantities and presence of important metabolites depend on the various parts of the plant used. Therefore, depth study of Tribulus L. medicinal ingredients and flavonoids can provided the basis for further development and utilization.
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML