-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2012; 2(1): 6-12
doi: 10.5923/j.ijmb.20120201.02
Evaluation of Extract of Ficus Exasperata Vahl Root Bark for Antimicrobial Activities Against Some Strains of Clinical Isolates of Bacterial and Fungi
Lawal I. O. 1, Borokini T. I. 2, Oyeleye A 1, Williams O. A 1, Olayemi J. O. 3
1Forestry Research Institute of Nigeria(FRIN), Ibadan, Nigeria
2National Centre for Genetic Resources and Biotechnology(NACGRAB), Ibadan
3Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan. Nigeria
Correspondence to: Lawal I. O. , Forestry Research Institute of Nigeria(FRIN), Ibadan, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ficus exasperata Vahl a small tree well known on account of its very rough leaves being used as paper widely spread in all eco-regions of Nigeria. The plant has been ethnobotanically reported to have diverse medicinal uses. It is used in herbal medicine to treat cough, hemorrhoid and lowering high blood pressure. Methanol and Ethylacetate extract of this plant were obtained by maceration and tested for antimicrobial activity using agar diffusion and micro broth method dilution techniques. The extracts were tested against strains of S. aureus, E. coli, B. subtilis, Ps. aeruginosa and fungi including four species of Candida. The study showed that extracts of Ficus exasperata root bark exhibited profound antibacterial activity against the typed and clinical isolates. High antifungal activity, particularly against the Candida species was noted as Candida species are implicated in candidiasis and vaginal thrush. Generally, the methanol extract was more effective than the ethylacetate extracts on the test micro-organisms. Minimum Inhibitory Concentration(MIC) of the extract was determined However, Phytochemical analysis of the root bark revealed the presence of saponin and cardiac glycoside which is an indication of medicinal significance of the root bark of Ficus exasperata. This study justifies the ethno pharmacological uses of these medicinal plants for treatment of microbial infections.
Keywords: Ficus Exasperata Vahl, Antimicrobial, Phytochemical Screening
Article Outline
1. Introduction
- Infectious diseases account for approximately one-half of all deaths in tropical countries. Worldwide, infectious diseases is the number one cause of death, and death from infectious diseases, ranked 5th in 1981 has become the third leading cause of human death in 1992, an increase of 58% (Pinner et al, 1996). The increase of microbial infections have increased dramatically in the past 20 years because of the increase in the number of people whose immune systems are compromised by AIDS, aging, organ transplant and cancer therapy. Accordingly, increase in the rates of morbidity and mortality because of microbial infections have been regarded as a major problem (Tatli & Akdemir, 2005).Worse still, there are global problems of multiple antibiotics resistance as well as emergence of new and resurrection of previously eradicated diseases. Most of the current antimicrobial drugs simply reduce the level of growth of bacteria or fungi, and some of them are very toxic to the kidney, the hematopoietic and central nervous system (Tatli & Akdemir, 2005). Antimicrobial resistance among enteric pathogens is becoming a matter of serious concern (El-Mahmood et al, 2008) and poses a great threat to global human health. Further, new microbial strains are being continuously discovered, which are refractory to the current arsenal of drugs (Erturk et al, 2006). This is because antimicrobial resistance leads to therapeutic failures of empirical therapy (Parekh & Chanda, 2007). As a result, it has become necessary to fight against emerging and re-emerging infectious diseases with a view to discover and invent new agents of greater therapeutic profile to mitigate frequent outbreaks of diseases which has posed a new threat to global health security (Mohanta et al, 2007). The continuous evolution of bacterial resistance to currently available antibiotics has necessitated the search for novel and effective antimicrobial compounds (Fagbemi et al, 2009), to which these micro-organisms are yet to develop resistance (El-Mahmood et al, 2008).With the rising problems of side effects and limited efficacy of antibiotic drugs (Gupta et al, 1998), there is an urgent need for the development of alternative antimicrobial substances and researchers are nowadays turning to natural products from plants (Nitta et al, 2002), as their main source of bioactive compounds with antimicrobial properties, to complement the existing synthetic antimicrobial drugs that are gradually becoming less potent against pathogenic micro-organisms.The use of medicinal plants all over the world predates the introduction of antibiotics and other modern drugs into Africa (Haslam et al, 1989). Medicinal plants constitute an effective source of antimicrobial natural products (Haslam et al, 1989). Historically, plants have provided a source of inspiration for novel drug compounds, as plant-derived medicines have made large contributions to human health and well-being. Their role is two-fold in the development of new drugs: (a) they may become the base for the development of a medicine, a natural blueprint for the development of new drugs, and (b) a phytomedicine to be used for the treatment of diseases (Iwu et al, 1999). It is estimated that today, plant materials are present in or have provided the models for 50% western drugs (Robbers et al, 1996).Phytomedicine have demonstrated its contribution to the reduction of excessive mortality, morbidity and disability dues to diseases such as HIV/AIDS, malaria, tuberculosis, sickle cell anaemia, diabetes, mental disorders (Elujoba et al, 2005) and microbial infections (Iwu et al, 1999). In addition, phytomedicines are effective in treating infectious diseases as well as limit side effects associated with synthetic antimicrobial drugs (Iwu et al, 1999). Many diseases have been handled traditionally and these include diarrhea, dysentery, flatulence, malaria, infantile convulsions, tonsillitis, bacterial and fungal infections and worm infestation (Ogueke et al, 2006). Medicinal uses of plants range from the administration of the roots, barks, leaves and seeds to the use of extracts and decoctions from the plants (Sofowora, 1982, Ogbulie et al, 2004). Phytomedicines have multiple effects on the body. Their actions often go beyond the symptomatic treatment of diseases. An example of this is Hydrastis canadensis (golden seal) which has not only antimicrobial activity, but also increases blood supply to the spleen, promoting optimal activity of the spleen to release mediating compounds (Murray, 1995).Plants have a diverse range of bioactive molecules, making them rich sources of different types of medicines (Nair et al, 2005). These bioactive compounds, according to Aboaba et al (2006), usually interfere with the growth and metabolism of micro-organisms in a negative manner. Furthermore, Aboaba & Efuwape (2001) reported that many plants contain non-toxic glycosides that can get hydrolyzed to release phenolics that are toxic to microbial pathogens. They stated further that saponins have antifungal properties.Screening of medicinal plants for antimicrobial agents has gained much importance in the development and utilization of medicinal plant resources in the traditional system of medicine in the developing countries so as to extend the health care to maximum number of population in these countries (Goud et al, 2005). In addition, screening of compounds obtained from plants for their pharmacological assays has indeed been the vast source of innumerable therapeutic agents represented by molecular diversity engineered by nature (Mohanta et al, 2007). Therefore, plants around us can be investigated for the purpose of identifying those that may be potent against infectious organisms and hence, useful in treating ailments caused by micro-organisms (Akinpelu et al, 2009).Ficus exasperata belongs to the family Moraceae, and is commonly called sand paper tree/plant, widely spread in all eco-regions of Nigeria. The plant has been ethnobotanically reported to have diverse medicinal uses. The leaf extract is reported to have diverse medicinal uses such as treating hypertensive patients (Buniyamin et al, 2007), haemostative, ophthalmia, coughs and haemorrhoids (Odunbaku et al, 2008). The root bark is reported to be used in the treatment of high blood pressure (Lawal et al, 2009). The leaf is used to scratch skin parts affected by ringworm while the grounded leaves applied topically are used to treat boils (Okoli et al, 2007). Furthermore, the young leaves are prescribed as a common anti-ulcer remedy (Adebayo et al, 2009). Various pharmacological actions such as anti-diabetic, lipid lowering and anti-fungal activities have been reported for Ficus exasperata (Sonibare et al, 2008). Other industrial uses of sand paper leaves are for polishing woods (Cousins & Michael, 2002), stabilization of vegetable oils, suppression of foaming, supplement as food stock and antimicrobials (Odunbaku et al, 2008). The activities of the leaf extract of Ficus exasperata against some pathogenic organisms have been extensively investigated (Buniyamin et al, 2007; Odunbaku et al, 2008). Furthermore, it was reported that the viscid non-milky sap is used for treating sores eye trouble and stomach pains in Ivory Coast (Burkill, 1997). The sap is used to arrest bleeding in Ghana (Abbiw, 1990). The liquid in which the bark is boiled is given to cows to hasten the expulsion of the after birth (Hallan, 1979). It is also used by traditional birth attendants (TBAS) in Congo to ease childbirth (Bouquet, 1969). In Southern Africa scrapings of the bark is used in an embrocating of the body and also as a stimulant (Burkill, 1997). In Upper Ivory Coast it is applied to leprous sores (Bouquet 1969). In Zaire a leaf poultice is used in medication for ring worm (Burkill, 1997). Chest complications are treated in the Gambia by steam inhalation of the leaves boiled in water.With these prior knowledge on the recent microbial resistance to antibiotics and the opportunity to produce new potent antimicrobial drugs from plants, this study is conducted to evaluate the antimicrobial effects of the methanolic and ethyl acetate extract of the root bark of Ficus exasperata on eight bacterial and nine fungal species; and as well screen the plant extract for the presence of secondary metabolites.
2. Methodology
2.1. Plant Materials
- Fresh root bark of Ficus exasperata was collected in the Herbal garden of Forestry Research Institute of Nigeria (FRIN), Ibadan in the month of May, 2009 and was identified at the Forestry Herbarium (FHI), Ibadan. The root bark samples was air dried and grounded to a powdery form using hammer mill. and kept for analysis
2.2. Preparation of the Extracts
- 100g of the powdered root bark sample was macerated with 100mL of 90% methanol (MeOH) for 72 hours and pooled to obtain the crude methanol extract. Same method and procedure was used to obtain the crude ethyl acetate extract of Ficus exasperata root bark.
2.3. Target Micro-organisms
- The test micro-organisms used for this study are bacteria and fungi strains. The bacterial species are Bacillus subtilis, Pseudomonas aeruginosa, three stains of Staphylococcus aureus and three strains of Eschericha coli. The fungal species used for the study are Candida albicans, Candida krucei, Candida glaberata, Candida tropicalis, Aspergillus niger, Aspergillus flavus, Colletotrichum gloesporoides, Trichoderma asperelum and Fusarium species. The micro-organisms were collected from Departments of Pharmaceutical Microbiology, Veterinary Microbiology, University of Ibadan and the International Institute of Tropical Agriculture (I.I.T.A) Ibadan.
2.4. Phytochemical Screening
- Screening for presence of secondary metabolites was performed following standard micro-chemical tests (Harborne and Harborne, 1998; Evans, 2002).
2.5. Antimicrobial Assays
- The agar well diffusion method was adopted for screening for antibacterial activity (Reeves et al., 1979; Okeke et al, 2001).O.1ml of a 1 in 100 dilution of the overnight broth culture of each bacterium (106–107 viable cells [cfu]) per mL of culture medium, determined by McFarland Nephelometry (NCCLS, 1993), was used to seed sterile molten nutrient agar maintained at 450C. The plates were allowed to solidify. 8mm diameter wells were bored in the seeded plates. Concentrations of 12.5mg/ml, 50mg/ml, 100mg/ml and 200mg/ml (dissolved in 50% MeOH) of each plant extract were added into appropriate wells and allowed to stand for two hours at room temperature to diffuse before incubating at 37OC for 24 hours.Gentamycin (10μg/ml) dissolved in 50% MeOH was used as positive control while 50% MeOH was used as negative control (no inhibition). All experiments were performed in triplicate. Antifungal tests were performed in a similar manner but seeded with appropriate fungi hyphae in saboraud dextrose agar, except that Candida albicans was inoculated in malt extract broth (Abo et al, 1998; Abo et al, 1999). All plates were subsequently incubated at room temperature for 96 hours. Griseofulvin (10μg/ml) dissolved in 50% MeOH was used as reference antifungal drug. The mean diameter of zones of inhibition (mm) was measured as basis for activity. The minimum inhibitory concentration (MIC) was determined by macrobroth dilution technique as previously reported (Sahm and Washington, 1990; Okoli and Iroegbu, 2003; Abo and Olugbuyiro, 2004). The tube with the lowest dilution with no detectable growth was considered as the MIC (El-Mahmood et al., 2008).
3. Results
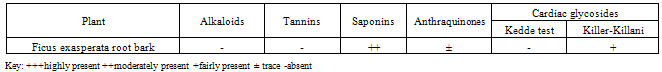
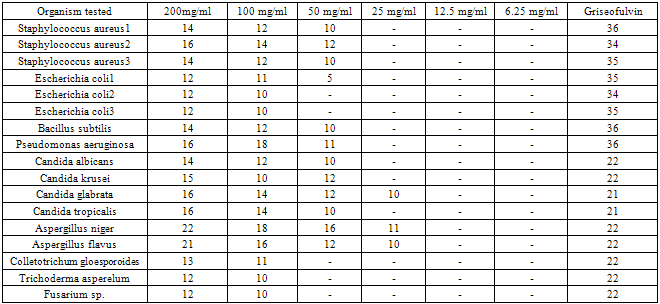
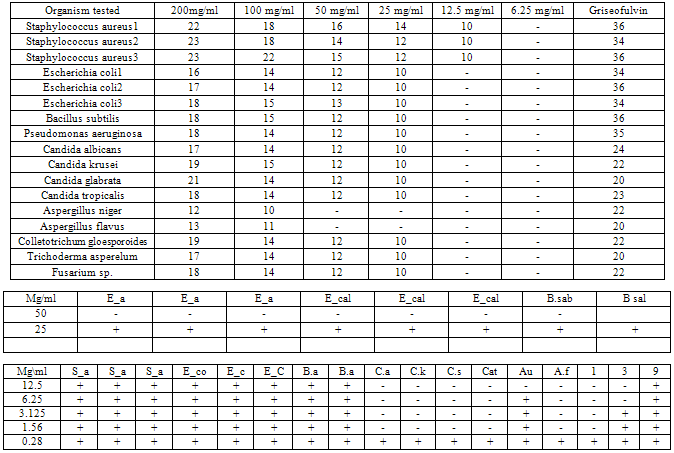
- Investigations on the phytochemical screening of the root bark extract of Ficus exasperata, as stated on table 1, indicated the presence of saponins, cardiac glycosides and anthraquinones in faint quantity, while tannins and alkaloids are absent. However, previous studies on the phytochemical analysis of Ficus exasperata by Adebayo et al (2009) showed that the root contained tannins, flavonoids, saponins, phlobatanins, glycosides and steroids.Tables 2 and 3 present the calculated mean zones of inhibition (mm) of ethyl-acetate and methanolic extracts of Ficus exasperata root bark on the bacterial and fungal strains used for this study respectively. The mean zones of inhibition of growth of the isolates are a function of relative antibacterial and antifungal activities of the extracts. The zone of inhibition is simply the area on the agar plate that remains free from microbial growth. The size of the zone of inhibition is usually related to the level of antimicrobial activity present in the sample or product - a larger zone of inhibition usually means that the antimicrobial is more potent.The extracts showed selective levels of activities against the isolates. On table 2, it was observed that the potency of the root bark extract of Ficus exasperata reduced with decrease in the concentration. The highest rates of inhibition were observed across all the micro-organisms tested at 200mg/ml, while concentrations 12.5mg/ml and 6.25mg/ml recorded no antimicrobial potency, while concentration 25mg/ml recorded inhibition of microbial growth for only three fungal strains. The potency of the ethyl-acetate extract of Ficus exasperata root bark was the highest on Aspergillus niger (22mm), followed by Aspergillus flavus (21mm) both of which are fungal strains. The highest inhibition against bacterial growth was recorded for Staphylococcus aureus2 (16mm). Generally, it appears that the growth of fungal strains is inhibited the more than the bacterial growth under the same treatment with ethyl-acetate extract.The results on table 3, however, vary considerably from that of table 2. The methanolic extract of the root bark of Ficus exasperata indicated the highest inhibition growth zone in Staphylococcus aureus2 and Staphylococcus aureus3 (23mm each), followed by Staphylococcus aureus1 (22mm) at 200mg/ml extract concentration. Among the fungal strains, the growth of Candida glabrata was inhibited most (21mm) at 200mg/ml extract concentration. Similar to the results expressed in table 2, the inhibition growth zone reduced as the concentration of the methanolic extracts decreased. The extract was potent against only three microbial strains (Staphylococcus aureus1, Staphylococcus aureus2, Staphylococcus aureus3) at 12.5mg/ml, while at 6.25mg/ml, no potency or inhibition was recorded. Studies by Adebayo et al (2009) indicated that the methanolic extract of Ficus exasperata root recorded a high rate of inhibition of microbial growth of Escherichia coli, Salmonella typhi and Vibrio cholerae, which are responsible for causing stomach illnesses. They further reported the relatively large zone of inhibition by the methanolic extract of the root against Pseudomonas aeruginosa, which corresponds to the findings of this work. This suggests that the plant can be used in the treatment of the diseases caused by this microorganism. A downward trend in the potency of the extracts on the inhibition of the microbial growth corresponding to the decreasing extract concentration was also observed in the work done by Adebayo et al (2009).
|
|
|
4. Discussions
- The need to develop new antimicrobial agents and antibiotics stems from the fact that microorganisms are developing resistance to many drugs and the death rate from infectious diseases have increased tremendously Plants have long been used for the treatment of infectious diseases such as asthma, sexually transmitted infections, skin infections and many others. The medicinal value of these plants lies in some chemical substances that produce a definite physiological action on the human body (Edeoga et al, 2005).This study has revealed that the extracts from Ficus exasperata root bark have antimicrobial effects on the clinical strains of the seventeen micro-organisms used for this study. The antifungal effect of the root bark is noted, especially against Aspergillus niger and Aspergillus flavus. In addition, antibacterial properties of the root bark against Staphylococcus aureus is also worth mentioning. This could be interpreted to mean that the root bark of Ficus exasperata may be relevant in the treatment of the diseases caused by Aspergillus niger, Aspergillus flavus and Staphylococcus aureus.In addition, it should be noted that comparatively, methanolic extracts of Ficus exasperata root bark have greater potency against the tested micro-organisms than the ethyl-acetate extracts. Furthermore, 25mg/ml concentration of ethyl-acetate extract had no potency against the growth of any of the microbial strains while the methanolic extract had high inhibition rate against the microbial growth of all, except two of the microbial strains used for the study. This indicates that the methanolic extract of the root bark is more effective against microbial growth than the ethyl-acetate extract. The results of these inhibition growth zones of the extracts of Ficus exasperata is a confirmation of the ethnomedicinal significance of the plant, especially against fungal infections on the skin.Furthermore, the presence of saponins and cardiac glycosides is an indication of medicinal significance of the root bark of Ficus exasperata. Saponins are glycosides of both triterpenes and steroids having hypotensive and cardiac depressant properties, and have been detected in over seventy plant families (Basu & Rastogi, 1967, Olaleye, 2007). They have been shown to possess beneficial properties by lowering the cholesterol level, have anti-diabetic and anti-carcinogenic properties (Trease and Evans, 1989) as well as being used as an expectorant and emulsifying agent (Edeoga et al, 2006). In addition, Cardiac glycosides are cardioactive compounds belonging to triterpenoids class of compounds (Brian et al, 1985). Their inherent activity resides in the aglycone portions of their sugar attachment. Their clinical effects in cases of congestive heart failure are to increase the force of myocardiac contraction (Brian et al, 1985). They exert their hypotensive effect by inhibiting Na+-K+ ATPase. Cardiac glycoside acts on the heart muscles and increase renal flow (diuresis). They also act directly on the smooth muscle of the vascular system. They exert a number of effects on neural tissue and thus indirectly influence the mechanical and electrical activities of the heart and modify vascular resistance and capacitance (Olaleye, 2007).Conclusively, this study shows that Ficus exasperata is effective against the test pathogens and it justifies the ethno pharmacological uses of both plants in the treatment of microbial infections. Further investigations will be conducted on these medicinal plants to ascertain the active antimicrobial bioactive compounds of this plant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML