-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2024; 12(1): 1-5
doi:10.5923/j.ijim.20241201.01
Received: Oct. 29, 2024; Accepted: Nov. 22, 2024; Published: Dec. 11, 2024

Case Report on Snakebite Envenoming in a Rural West African Snake Hunter
Rex K. Bonsu1, Nana A. B. Owusu Sekyere2, Maame D. Boateng3
1Department of Medicine and Therapeutics, Korle-Bu Teaching Hospital, Accra, Ghana
2Department of Internal Medicine, University Hospital, Legon, Ghana Health Service
3Department of Surgery, Korle-Bu Teaching Hospital, Accra, Ghana
Correspondence to: Rex K. Bonsu, Department of Medicine and Therapeutics, Korle-Bu Teaching Hospital, Accra, Ghana.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

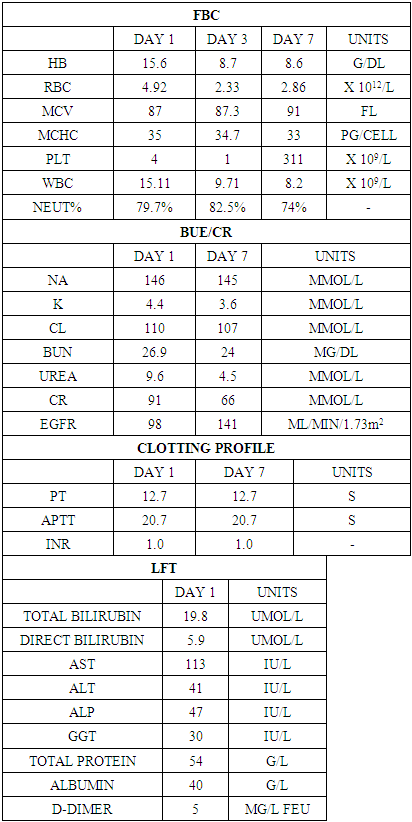
Snakebite envenoming (SBE) is a major public health issue in rural West Africa, predominantly affecting male agricultural workers. Species from the Elapidae and Viperidae families often inflict SBE. It can cause disseminated intravascular coagulation (DIC), characterized by varying degrees of coagulation activation, from minor decreases in platelet count and clotting time prolongation to severe microvascular thrombosis and bleeding. Case Presentation: A 59-year-old snake hunter presented to the emergency room shortly after a viper bite to his left finger. He experienced brief loss of consciousness and rapidly progressing symptoms, including left-hand swelling and back ecchymosis. Examination revealed soft tissue destruction, bruises, and swelling on the left hand, forehead, and back, but no visible fang marks. He exhibited gross hematuria, gum bleeding, and bleeding from IV puncture sites. He was in shock, with a pulse rate of 120 bpm and blood pressure of 70/43 mmHg. Initial resuscitation included IV hydration, polyvalent antivenom, steroids, tetanus vaccine, antibiotics, and blood products. The bleeding resolved after three days. Debridement and supportive care continued, with rapid clinical improvement. He was discharged after three weeks, with counseling on occupational safety. Clinical Discussion Following a snakebite, initial symptoms may be minimal, but systemic symptoms can rapidly worsen. Though other clotting parameters may be normal, Platelet count and D-dimer levels are sensitive severity indicators for DIC. Prompt assessment and management are crucial, especially in low-resource settings, to prevent fatalities. Conclusion: Rapid deterioration is common; anticipating complications and applying proper management principles significantly reduces mortality.
Keywords: Snakebite venom, Disseminated intravascular coagulation (DIC), Clotting profile, Africa, Case Report
Cite this paper: Rex K. Bonsu, Nana A. B. Owusu Sekyere, Maame D. Boateng, Case Report on Snakebite Envenoming in a Rural West African Snake Hunter, International Journal of Internal Medicine, Vol. 12 No. 1, 2024, pp. 1-5. doi: 10.5923/j.ijim.20241201.01.
1. Introduction
- An estimated 5.4 million snake bites occur across the globe yearly, with 1.8 to 2.7 million envenoming [1]. Case fatality rates range from 81,400 to 137,880 yearly, with about three times amputations and other permanent disabilities occurring [1]. There is limited research on the prevalence of snakebites in Ghana especially in urban areas. However, one study reports an average of 9,600 snake bites yearly between 2015 and 2019 [2,3]. The issue remains a significant public health threat, with envenomation rates in rural areas ten times as high as rates in urban areas [4,5]. It is estimated that the rate of hospital visits by snake bite victims is 35/100,000 persons per year, and a case fatality rate of up to 11% has been reported in a pre-intervention study in a rural health facility in Ghana [6,7]. Elapidae and Viperidae species are common venomous snake species associated with severe envenoming in Ghana [8]. Envenomation from neurotoxic venom-expressing snakes, typically Elapids, may include these symptoms: vomiting, dizziness, blurred vision, muscle weakness with inability to walk, dysphagia, and paresthesia. Eye signs may include palpebral ptosis, ophthalmoplegia, and facial muscle weakness [8,9]. The Vipers elaborate venoms that are hemorrhagic, nephrotoxic, cardiotoxic, cytotoxic, and prothrombotic [8]. These venoms may cause local effects such as tissue swelling, pain, skin blisters, lymphangitis, and necrosis. Venoms also have systemic effects such as cardiovascular depression causing shock, acute kidney injury, and bleeding diathesis [8,10].Polyvalent anti-snake venoms available in Ghana contain anti-venoms against about 14 common species of vipers and elapids, making them more useful antidotes for snake bite envenomation [8].This case report describes a middle-aged snake hunter who developed DIC as a complication of snakebite envenomation from a viper, without the typical laboratory features of DIC and was successfully treated with several doses of polyvalent anti-snake serum, IV fluids, antibiotics, and several blood products.
2. Case Presentation
- A 59-year-old snake hunter presented to the Emergency Department (ED) of the Korle Bu Teaching Hospital with left upper limb swelling and pain five hours after being bitten by a viper on his left finger. He briefly lost consciousness and experienced progressive, painful swelling of his left hand. Three hours post-bite, the swelling had worsened, extending to his entire forearm with blisters and dark patches. He was initially taken to a nearby hospital and then referred to a larger facility for further treatment.He appeared acutely ill and dizzy but fully conscious. His urethral catheter drained bloody urine and was moderately pale, afebrile, and anicteric. His extremities were cold without pedal edema, and he had swelling and bruising on the left side of his forehead. He was bleeding from IV puncture sites and gums, had a weak and thready pulse at 120 bpm, and had a blood pressure of 70/43 mmHg. His heart sounds were normal without murmurs, his respiratory rate was 18 CPM, SpO2 was 98% on room air, and his chest was clear on auscultation. His left upper limb was swollen and tender with large bullous lesions extending from the hand to the elbow, areas of hyperpigmentation, and necrotic skin patches, though no fang marks were visible (Figure 1).
 | Figure 1. Image showing skin necrosis of the left arm post envenomation |
|
3. Discussion
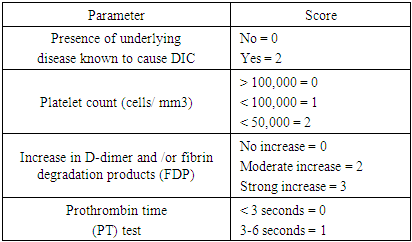
- Definitive diagnosis of snake venom poisoning requires either identification of the snake, assessment of the snake bite wound, or the clinical manifestations of envenomation [11]. Snake envenomation results in a myriad of complications, such as cytotoxicity, neurotoxicity, hemostatic complications, nephrotoxicity, myotoxicity, cardiotoxicity, anaphylaxis, etc [12]. Snake venoms are complex mixtures containing many different biologically active proteins, out of which a variety acts on the hemostatic system in humans. The following homeostatically active venomic enzymes have been studied - enzymes that cause fibrinogen coagulation; enzymes that degrade fibrinogen: plasminogen activator, prothrombin activators; factor V activator, factor X activator; anticoagulant activities; enzymes with hemorrhagic activity; platelet aggregation inducers; and platelet aggregation inhibitors. Alvarez-Florez et al. simplified hemostatic snake venoms as procoagulants (prothrombin activators, thrombin-like enzymes, and factor X and factor V activators) and anticoagulants (phospholipase A2, Protein C Activators, L-Amino Acid Oxidases, etc) [13].The consensual definition of DIC, as proposed by the Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH), is as follows: “DIC is an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes [16]. Disseminated Intravascular hemolysis results from the interplay of snake venom components, host responses, and coagulation pathway. The pathomechanism of DIC from snakebite envenomation can be summarized as:Direct Activation of Coagulation Cascade: Venom toxins, such as procoagulant enzymes and metalloproteinases, can directly activate components of the coagulation cascade, including Factor X, thrombin, and fibrinogen. This leads to the generation of thrombin and subsequent conversion of fibrinogen to fibrin, initiating intravascular clot formation [13]. Endothelial Dysfunction and Microvascular Damage: Snake venom toxins have been shown to induce endothelial cell injury and disrupt the endothelial barrier integrity. This endothelial dysfunction not only promotes procoagulant activity but also facilitates platelet adhesion and aggregation at the site of injury, further contributing to microvascular thrombosis [15]. Platelet Dysfunction and Consumption: Certain venom components can impair platelet function, leading to decreased platelet adhesion, aggregation, and secretion. Concurrently, the consumption of platelets within the forming thrombi exacerbates thrombocytopenia, perpetuating the cycle of coagulation abnormalities [16].Release of Proinflammatory Mediators: Snake venom contains a plethora of bioactive molecules that trigger inflammatory responses, including cytokines, chemokines, and prostaglandins. These proinflammatory mediators not only contribute to endothelial activation but also enhance the expression of tissue factors, further amplifying the coagulation cascade [17]. Clinically, patients typically present with local symptoms at the site of the bite, including pain, swelling, erythema, and tissue necrosis [18]. However, as the process continues, systemic manifestations become evident, reflecting the widespread activation of coagulation and consumption of clotting factors. The coagulopathy may include petechiae, purpura, ecchymosis, and mucosal bleeding. Patients may experience spontaneous bleeding from multiple sites, including gingiva, nose, gastrointestinal tract, and genitourinary tract. Hematuria and hematemesis may also occur, further giving credence to the severity of the coagulopathy [19,20]. Systemic symptoms such as fever, malaise, tachycardia, and hypotension may accompany DIC, reflecting the underlying inflammatory response and hemodynamic instability. Multiorgan dysfunction may ensue, particularly in severe cases, further exacerbating the clinical picture and prognosis. Diagnosis of DIC can be made from a combination of a clinical condition known to be associated with DIC and a series of laboratory results. Common laboratory abnormalities that support the diagnosis of DIC include decreasing order of frequency of thrombocytopenia, increased fibrin degradation products (D-dimer), prolonged PT, aPTT, and low fibrinogen levels [21]. Thrombocytopenia is seen in the majority (about 98%) of DIC patients, with platelet levels less than 50,000 seen in 50% of cases. A declining trend rather than a single measurement is most indicative and sensitive, even though not specific for DIC [22]. Elevations in titers of fibrin degradation products (D-dimer) should be assessed in the context of other relevant laboratory results, such as thrombocytopenia, prolonged PT, aPTT, etc, as it can be elevated in other conditions, too. However, normal D-dimer levels have a high negative predictive value as an indicator of intravascular fibrin degradation [23].About 50% of patients with DIC have prolonged PT and aPTT due to the consumption of coagulation factors. Also, about 50% of patients do have shortened PT and aPTT due to the presence of activated circulating factors such as thrombin. Therefore, while prolonged values in combination with other assays support the diagnosis of DIC, a normal or shortened PT or aPTT does not exclude its diagnosis. Repeated monitoring is the key [10,20].Hypofibrinogenemia for diagnosis of DIC has low sensitivity as its levels can remain elevated for prolonged periods as an acute phase reactant. Low fibrinogen levels are nearly present in about half of the patients with severe forms of DIC [10]. The diagnostic algorithm for overt DIC, as proposed by the Scientific Subcommittee on DIC of the International Society of Thrombosis and Hemostasis, ISTH, is described below in Table 2.
|
4. Conclusions
- This case report, written in line with the SCARE 2023 criteria [27], seeks to reveal the multifaceted nature of Disseminated intravascular coagulation, the effect of snake envenomation, and the life-threatening clinical consequences it poses to possible laboratory parameters.Clinical diagnosis should prompt appropriate management. Early treatment with polyvalent antisnake venom, supportive care, and monitoring accomplished by the healthcare providers indicates the advantageous course of the disease with timely and efficient action. Snake bites continue to remain a predominant public health threat hence public health strategies must be employed to help curb its prevalence. These strategies must focus on integrating efforts combining community education, safe practices and systemic improvements. Community education campaigns must emphasize the risks of snake bites and teach preventive measures such as wearing protective clothing during farming such as the use of boots and gloves. Enhancing access to antivenoms and healthcare infrastructure is essential on the systemic level. Despite their effectiveness, antivenoms are frequently unavailable or prohibitively expensive particularly in rural area. Training healthcare workers to recognize and treat snakebite envenomations and ensuring that facilities are equipped with appropriate anti-venoms can significantly improve outcomes. Additionally, Ghana's participation in the WHO's initiative to halve global snakebite mortality and disability by 2030 highlights the importance of coordinated efforts between local and international bodies. These combined efforts can reduce the burden of snakebites, which disproportionately affect rural, under-served communities in the country.
ACKNOWLEDGEMENTS
- I would like to thank the patient for consenting to the publication of this case report, as well as the medical personnel involved in the management of this case. Special thanks to my co-authors for their contributions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML