-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2022; 11(1): 54-58
doi:10.5923/j.ijim.20221101.03
Received: Feb. 13, 2022; Accepted: Feb. 28, 2022; Published: Mar. 15, 2022

Cryptogenic Organizing Pneumonia Obscured by a Presumed COVID-19 Pneumonia: A Case Report
Dylan Matthew Salazar1, Devanshi Narendra Damani2, Piya Kositangool1, Osvaldo Padilla3, Fatma Dihowm2
1Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso TX, USA
2Department of Internal Medicine, Texas Tech University Health Sciences Center El Paso, El Paso TX, USA
3Department of Pathology, Texas Tech University Health Sciences Center El Paso, El Paso TX, USA
Correspondence to: Dylan Matthew Salazar, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso TX, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Interstitial lung disease (ILD) is a group of more than 200 conditions with various etiologies that result in a wide range of inflammation and/or fibrosis of the pulmonary interstitium. Cryptogenic Organizing Pneumonia (COP) is a type of idiopathic ILD that requires a high index of suspicion with an appropriate workup to make the diagnosis. The Coronavirus Disease 2019 (COVID-19) pandemic has enabled COVID-19 pneumonia to become a top differential diagnosis, which has consequently introduced bias into medical decision-making. We present a case of COP that was misdiagnosed for COVID-19 pneumonia for months despite several negative COVID-19 test results. This case also raises awareness of the cognitive bias that likely allowed for the misdiagnosis, resulting in delayed appropriate treatment for this patient.
Keywords: Cryptogenic Organizing Pneumonia, COVID-19 Pneumonia, Diagnostic Error, Cognitive Bias, Availability Bias
Cite this paper: Dylan Matthew Salazar, Devanshi Narendra Damani, Piya Kositangool, Osvaldo Padilla, Fatma Dihowm, Cryptogenic Organizing Pneumonia Obscured by a Presumed COVID-19 Pneumonia: A Case Report, International Journal of Internal Medicine, Vol. 11 No. 1, 2022, pp. 54-58. doi: 10.5923/j.ijim.20221101.03.
1. Introduction
- The Coronavirus Disease 2019 (COVID-19) pandemic has dramatically impacted the healthcare system – from becoming a public health crisis to influencing the medical care of the individual patient [1]. The prevalence of COVID-19 has resulted in many providers becoming susceptible to availability bias when diagnosing and treating patients. In fact, availability bias is a type of cognitive bias that influences diagnostic decision-making. It is described as the tendency to determine a diagnosis based on how easily it can be recalled [2]. This has served as the predisposition to inaccurately attribute an organizing pneumonia being secondary to COVID-19 pneumonia – even in the absence of conclusive evidence. Organizing pneumonia is deemed an interstitial lung disease that entails two types: cryptogenic and secondary [3]. Cryptogenic organizing pneumonia (COP) is a rare type of idiopathic interstitial lung disease discerned histologically by alveoli and alveolar ducts filled with spindle-shaped fibroblasts and myofibroblasts, which can later form granulation tissue [4]. On the contrary, the etiology for secondary organizing pneumonia can be identified. COP is typically confirmed with lung tissue biopsy as it is challenging to diagnose clinically due to its rarity, low clinical suspicion, and nonspecific symptoms [3,4]. In this case report, we describe a case of misdiagnosing COP for COVID-19 pneumonia despite several negative test results, leading to a delay in the appropriate management of a young male. This case also raises awareness of the impact the current pandemic has made on medical decision-making.
2. Case Report
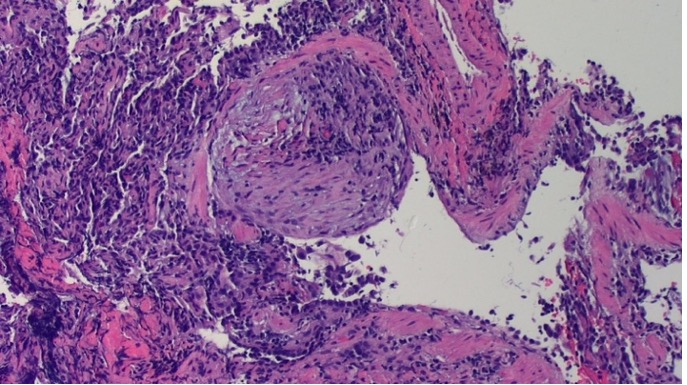
- A 36-year-old male with no comorbidities, a negative smoking history, and fully vaccinated with Pfizer vaccine for COVID-19 presented with worsening shortness-of-breath. Two months prior to presenting at our hospital, the patient reported the onset of symptoms shortly after donning inappropriate protective equipment while cleaning a walk-in cooler room contaminated with mold. The symptoms included a nonproductive cough, shortness-of-breath that worsened with exertion, and fatigue. The patient's symptoms progressively worsened meeting criteria to be admitted to a hospital. At that facility, the patient was hospitalized for 3 days and was discharged with a final diagnosis of COVID-19 pneumonia. At his follow-up visit with his primary care physician, he reported worsening shortness-of-breath with self-reported oxygen saturation levels measuring in the 80s, which was confirmed at the visit. Again, the patient was given the diagnosis of COVID-19 pneumonia, despite 4 negative COVID-19 test results consisting of both the rapid and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) tests. He was then prescribed a prophylactic 7-day course of Levofloxacin and 2 liters of supplemental home oxygen that ultimately failed to resolve his symptoms.On admission to our hospital, the patient presented with worsening shortness-of-breath, subjective fevers, and retrosternal chest pain that worsened with deep inhalation. He was initially admitted to the Medical Intensive Care Unit (MICU) due to his escalating oxygen requirement with an oxygen saturation of 80% on room air. Less than 24 hours in the MICU, the patient was accepted to the floor as he improved to only requiring 6 liters of oxygen on nasal cannula to maintain an oxygen saturation of at least 90%. While on the floor, the patient denied any chills, bloody sputum, nausea, vomiting, urinary symptoms, and lower extremity swelling. The patient had no significant past medical, surgical, or family history. He denied taking any regular medications and any recent travel within and out of the United States of America. He was employed as a convenient store manager and denied any substance use and owning any pets. His admitting vitals showed a blood pressure of 122/85 mmHg, pulse of 88 beats per minute, respiratory rate of 20 breaths per minute, temperature of 37.3°C, and oxygen saturations of 80% on room air, which was increased to 90% with appropriate supplemental oxygen. A pulmonary physical exam revealed crackles on all posterior lung fields, bronchial breath sounds with fine crepitations, increased vocal resonance and egophony. His heart sounds were normal with a regular rate and normal rhythm with no gallops or rubs. A complete blood count showed an elevated hemoglobin and hematocrit (H&H) at 18.6 G/DL (reference; 12 to 16) and 55.5% (reference; 38 to 47), respectively. A complete metabolic panel was within normal limits. A Lactate Dehydrogenase was elevated at 531 U/L (reference; 120 to 246). An Erythrocyte Sedimentation Rate (ESR) was within normal limits; however, a C-Reactive Protein (CRP) was elevated at 4.60 mg/dL (reference; 0 to 1). A urinalysis was normal. PCR for SARS-CoV-2 was negative. A D-Dimer was elevated at 0.65 ug/mL (reference; < 0.50). Arterial Blood Gases showed an elevated H&H, with remaining measures being within normal limits. Cardiac troponins were within normal limits. A Reparatory panel was negative. Work-up for infectious etiology was negative for Streptococcus pneumoniae, Coccidioides, Legionella pneumonia, QuantiFERON gold, Human Immunodeficiency Virus (HIV), Hepatitis Panel, and two sets of blood cultures. Autoimmune workup for Rheumatoid Factor, anti-cyclic citrullinated peptide (CCP), Anti-Nuclear Antibodies (ANA), anti-double stranded DNA, Antineutrophil Cytoplasmic Antibodies (ANCA), and anti-centromere antibody were negative. A Chest X-ray (CXR) displayed bilateral airspace consolidation with scattered ground-glass opacities in the apices. Computed Tomography (CT) of the chest revealed peripheral upper lobe ground-glass opacities with interstitial thickening in a crazy-paving pattern (Figure 1). A chest CT angiogram was positive for peripherally predominant patchy ground-glass pulmonary infiltrates consistent with severe COVID-19 pneumonia, and negative for a pulmonary embolism. An Electrocardiogram showed possible inferior myocardial infarction, prompting order of cardiac troponin levels that were within normal limits. A Transthoracic Echocardiogram was negative for any cardiac abnormality. Bronchoalveolar lavage (BAL) with culture was negative for acid fast bacilli, fungal organisms, Pneumocystis jirovecii, and malignant cells. Bronchoscopic biopsy of the left upper lobe was subsequently performed and revealed lung parenchyma with fibroblastic plugs of loose, myxoid collagen matrix that was found within alveolar and bronchiolar spaces. Lymphoplasmacytic infiltrates were also identified (Figure 2). These microscopic features are compatible with an organizing pneumonia.
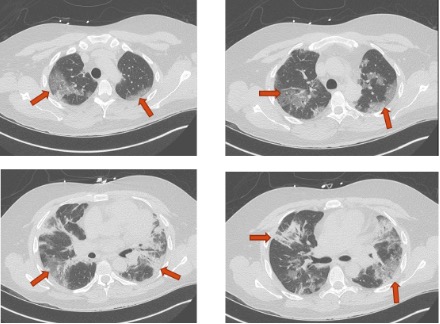 | Figure 1. Initial Chest CT on Presentation. Note the red arrows on image discern the crazy-paving pattern, which is described as ground-glass opacities with superimposed interstitial thickening |
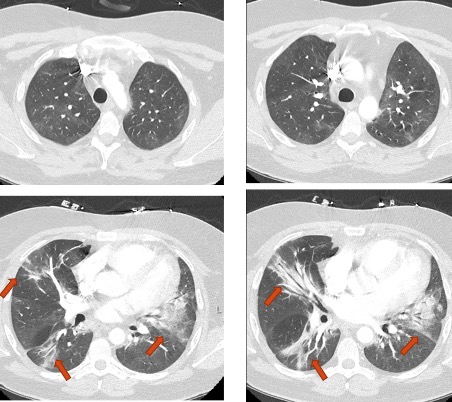 | Figure 3. Chest CT Angiogram 8 Weeks Status-Post High-Dose Steroid Treatment. Note the dramatic improvement in the severity of pulmonary infiltrates as compared to Figure 1 |
3. Discussion
- Cryptogenic organizing pneumonia is difficult to diagnose because it is a rare disease that often presents with nonspecific symptoms. COP typically presents in the fifth or sixth decade of life with both genders being equally affected. The classic history of present illness for COP entails a subacute onset of a persistent nonproductive cough, dyspnea, fever, fatigue, and weight loss. Hemoptysis has also been identified as an associated symptom of COP in some patients; however, its presence is not common [5]. Additionally, many patients will have a history of failing an antibiotic trial for a presumed bacterial pneumonia. Our patient presented with a similar clinical picture despite many of his symptoms being nonspecific and not meeting the classic age of onset. Laboratory workup for COP often reveals elevated inflammatory markers, specifically ESR and CRP. Considering our patient presented with pleuritic chest pain – which is also a rare symptom to express with COP – a d-dimer was assessed, yielding positive results. In fact, d-dimers have shown to be elevated in patients with respiratory symptoms and non-pulmonary embolism lung pathologies like COP, malignancy, and infections [5]. Like many other lung diseases, imaging studies are essential to complete the diagnostic workup for COP. CXR findings for COP tend to show bilateral patchy or diffuse consolidation with ground-glass opacities – consistent with the CXR performed on our patient. CT scan of the chest typically shows patchy air-space consolidations and ground-glass opacities with bronchial wall thickening – similar to the CT findings in our patient [5,6]. Because COP is an idiopathic interstitial lung disease, all other etiologies must be ruled out before confirming its diagnosis. There are many types of additional investigations that must be performed to confirm the idiopathic nature of the disease. For example, performing a bronchoscopy with bronchoalveolar lavage is useful to exclude malignancy and infectious organisms. Of note, it is not uncommon for BAL to show nonspecific inflammatory patterns in COP patients [5]. Nonetheless, the ultimate diagnosis of COP is accomplished by lung biopsy along with correlated clinical and radiological findings. Treatment with oral corticosteroids can result in prompt improvement of symptoms with resolution of opacities on radiographs – as observed in the outcome of our patient. Relapses tend to occur with abrupt discontinuation of corticosteroids; therefore, long-term steroid treatment calls for close clinical observation. There are currently no established guidelines for an optimal treatment regimen of COP [5,6].The current COVID-19 pandemic has enabled COVID-19 pneumonia to become a top differential diagnosis, resulting in a complete overshadow of other etiologies with similar presentations. COVID-19 has a wide array of clinical manifestations – ranging from asymptomatic presentations to severely critical illness. The most common symptoms being reported for COVID-19 pneumonia include fever, dry cough, shortness-of-breath, sore throat, anosmia, dysgeusia, and chest discomfort. Laboratory investigations tend to vary and are currently still under investigation; however, most findings are consistent with an inflammatory process. These laboratory findings include, but are not limited to, leukocytosis, elevated CRP and ESR, elevated procalcitonin, and elevated d-dimer levels. Imaging studies can show a CXR displaying bilateral multifocal opacities within the alveoli, with or without pleural effusions. Multifocal bilateral ground-glass opacities seem to be one of the most common findings on a chest CT scan. It is important to note that our patient did display several of these features on both his labs and imaging. To definitively diagnose a patient with COVID-19 pneumonia, it recommended to use Reverse Transcription-Polymerase Chain Reaction as this has remained the gold standard for diagnosing COVID-19 throughout this ongoing pandemic [7-10].Even though our patient had several negative COVID-19 test results before presenting to our facility, many of his laboratory findings and imaging studies supported a diagnosis of severe COVID-19 pneumonia while at our hospital. This imbued a sense of concern for the possibility of a false-negative COVID-19 test result, which has been reported in many cases throughout this pandemic [11]. To be deemed a false-negative test result, a patient must have had a negative test result followed by a positive test result on repeat diagnostic testing. This bolstered our intuition to repeat a RT-PCR test to detect for SARS-CoV-2, which was negative as mentioned in the preceding section. The decision to obtain another RT-PCR test follows the recommendation of performing a repeat diagnostic test if there is suspicion for COVID-19 pneumonia despite negative test results [8,9,11].As the possibility for a false-negative COVID-19 test result was significantly minimized, it is important to discuss the impact the COVID-19 pandemic has made on medical decision-making. With COVID-19 pneumonia becoming a top differential diagnosis, this has increased the likelihood for diagnostic error among providers for several reasons. These reasons include the novel characteristics of COVID-19-related disease, physical and psychological burnout experienced by providers, and newly designed protocols that may be prone to error [12]. In a study aimed at reducing the risk of diagnostic error throughout the COVID-19 pandemic, Gandhi and Singh proposed a new taxonomy to define several types of diagnostic error expected to be seen throughout the pandemic. Notably, this classification solely entails diagnostic errors that are secondary to the impact COVID-19 has had on healthcare providers and the healthcare system [12,13]. Using this new typology, Shen uncovered that approximately 0.7% of all safety reports filed between March 1, 2020, and February 28, 2021, at an academic tertiary care referral center in Northeastern United States were due to COVID-19-related diagnostic error [13]. Currently this is one of the few studies assessing the impact the COVID-19 pandemic has made on diagnostic error rates. This is likely due to the greater emphasis placed on the prompt diagnosis and treatment of COVID-19 disease. This calls for further exploration of this detriment experienced by many patients. Regarding our case, the patient underwent a diagnostic error as he was still considered to have an underlying COVID-19 infectious etiology despite multiple negative test results. In fact, one of the most common and costly reason for malpractice claims is diagnostic error. These errors are typically multifactorial; however, the inaccuracy of diagnostic reasoning is noted in approximately 75% of these mistakes. This is largely attributed to the use of heuristics in medical decision-making to increase efficiency; however, heuristics frequently introduce several types of bias. Specifically, availability bias is when an incorrect diagnosis is more accessible to the mind [14]. Unfortunately, our patient experienced a diagnostic error likely due to availability bias that led to the delayed appropriate medical management for a COP diagnosis. Although an availability bias likely occurred with our patient’s case, it is important to recognize that both COP and COVID-19 pneumonia share several similarities in both presentation and investigative workup. For example, shared features from the history and physical exam include complaints of fever, malaise, cough, and shortness-of-breath. Laboratory results for both diseases display an elevated white count with neutrophil predominance, as well as elevated inflammatory markers like CRP and ESR. On imaging, CXR reveals bilateral, diffuse, patchy consolidations with associated pleural effusions. Additionally, high-resolution CT (HRCT) of the chest shows bilateral, asymmetric, patchy, peripherally located ground-glass opacities, a crazy paving pattern, pleural effusions, cavitations, and the classic reverse halo sign, which is described as a focal ground-glass opacity encompassed by a dense outer rim of consolidation [3,6,15,16]. Despite the similarities shared between the COP and COVID-19, there are also some differences that can help distinguish the diseases. For example, the symptoms of COP have a subacute onset, whereas COVID-19 symptoms have an acute onset. Moreover, COVID-19 can be deemed a systemic viral disease that affects many organs outside of the lungs [6,7].This case has shed light on the importance of implementing an intervention that counteracts availability bias among providers. Mamede et al. designed an experimental intervention aimed at refining the ability of physicians to discern unique features of diseases that present similarly for the purpose of avoiding bias-based influences. This study “immunized” physicians by increasing their knowledge of clinical features that help distinguish between similar-looking diseases. This immunization process transpired one week prior to administering an assessment composed of clinical vignettes known to introduce bias. This experiment was successful in reducing the susceptibility to bias; hence, reducing the rate of diagnostic errors [14]. Mamede also suggests that implementing a more profound reflective diagnostic reasoning approach, which entails methodically listing all pertinent findings that support and do not support a diagnosis, would further enhance diagnostic accuracy [2]. Both interventions would have likely prevented the delay in appropriate medical management, as well as reduction in the morbidity experienced by our patient. This bolsters the need for more investigative studies on interventions intended to reduce the rate of availability bias for the sake of appropriate patient care and management.
4. Conclusions
- About half of all patients with COP undergo spontaneous resolution [17]. Despite this high regression rate, treatment should be started promptly if suspicion of COP with supportive evidence arises. Treatment can be initiated devoid of lung biopsy, especially if the risks of an invasive lung biopsy outweigh the benefits [6]. For future COP cases, it is important to have a high index of suspicion for the diagnosis as it might be obscured by the prevalence of COVID-19 pneumonia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML