-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2022; 11(1): 47-53
doi:10.5923/j.ijim.20221101.02
Received: Feb. 13, 2022; Accepted: Feb. 28, 2022; Published: Mar. 15, 2022

Dyslipidemia in Patients with COVID-19 in Togo
Abdou Razak Moukaila1, Lidaw Deassoua Bawe2, 3, Komi Edem Mossi1, Komi Dzidzonu Nemi1, Awereou Kotosso2, 3, Tsevi Yawovi Mawufemo4, Agbeko Kodjo Djagadou1, Malewe Kolou5, Majeste Ihou Wateba2, Awalou Mohaman Djibril1
1Department of Internal Medicine, Sylvanus Olympio University Hospital, University of Lomé, Lomé, Togo
2Department of Infectious Diseases and Tropical Diseases, Sylvanus Olympio University Hospital, University of Lomé, Lomé, Togo
3Regional Hospital Center of Lomé-Commune, Togo
4Department of Nephrology and hemodialysis, Sylvanus Olympio University Hospital, University of Lomé, Lomé, Togo
5Department of Biology and Medical Biochemistry, Sylvanus Olympio University Hospital, University of Lomé, Lomé, Togo
Correspondence to: Abdou Razak Moukaila, Department of Internal Medicine, Sylvanus Olympio University Hospital, University of Lomé, Lomé, Togo.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Purpose: The severity of COVID-19 is associated with comorbidities such as hypertension, diabetes, and obesity, which are also well known cardiovascular factors. Dyslipidemia, although also cardiovascular risk factors, seems to have been less explored in terms of its role in the severity and prognosis of COVID-19, particularly in sub-Saharan Africa and especially in Togo. Method: We conducted a monocentric, cross-sectional, descriptive and analytical study at the Regional Hospital of Lomé-Commune. Dyslipidemia was defined according to NCEP-ATP III criterias. Results: Our study included 160 patients. Dyslipidemia was identified in 80.5% of cases. HDL hypocholesterolemia was the most represented dyslipidemia (45.7%) followed by hypertriglyceridemia (43%). Hypertriglyceridemia (OR = 2.1; CI: 1.1 - 4.1; p = 0.021), TC/HDL ratio≥5 (OR = 5.8; CI: 1.5 - 23.11; p = 0.011) and TG/HDLc ratio≥4 (OR= 10.1; CI: 3.4-30.1; p<0.001) are the factors associated with a greater risk of COVID-19 severity. Conclusion: Dyslipidemia during COVID-19 is very common in Togo and could play a significant role in the severity and mortality associated with COVID-19.
Keywords: Dyslipidemia, COVID-19, Triglyceridemia, TG/HDL-c ratio, HDL-c, Togo
Cite this paper: Abdou Razak Moukaila, Lidaw Deassoua Bawe, Komi Edem Mossi, Komi Dzidzonu Nemi, Awereou Kotosso, Tsevi Yawovi Mawufemo, Agbeko Kodjo Djagadou, Malewe Kolou, Majeste Ihou Wateba, Awalou Mohaman Djibril, Dyslipidemia in Patients with COVID-19 in Togo, International Journal of Internal Medicine, Vol. 11 No. 1, 2022, pp. 47-53. doi: 10.5923/j.ijim.20221101.02.
Article Outline
1. Introduction
- The COVID-19 pandemic has undeniably marked the end of the 2010-2020 decade. Indeed, the impact of the COVID-19 pandemic on health systems, social habits and economic activities has been unprecedented.As of September 7, 2021, WHO reported 221,134742 cases of Covid-19, including 4,574089 deaths worldwide since the pandemic began in December 2019. In Africa, 5,740184 cases have been counted with 138,005 deaths [1]. For the same period, Togo reported 22625 confirmed cases with 197 deaths recorded [2].The clinical presentation of COVID-19 varies from asymptomatic forms to forms of severe acute pneumonia that can be complicated by multivisceral failure, especially in elderly patients with chronic comorbidities [3,4]. COVID-19 can also be subject to cardiovascular manifestations such as myocarditis, cardiac rhythm disorders, acute coronary syndromes and thromboembolic diseases [5].Based on the epidemiological data available to date, the factors associated with the clinical severity of COVID-19 appear to be age, gender and comorbidities [6–8]. With few exceptions, most deaths are associated with underlying comorbidities [9]. Thus, several studies have reported the association between chronic obstructive pulmonary disease [10], asthma [11] or smoking [12] and the severity of COVID-19. Also, it has been reported that patients with underlying cardiovascular disease or cardiovascular risk factors had a high risk of more severe disease progression or mortality from COVID-19 [13,14].Lipids are crucial in the infection process as they are important structural components of cell membranes [15]. Although dyslipidemias are among the cardiovascular risk factors, the association between lipid fraction disorders and COVID-19 prognosis has been less frequently studied. In regard to this, a recent review of the literature, reported that dyslipidemias may play a role in the severity of SARS-CoV-2 infection [16].Given the magnitude that the COVID-19 epidemic has had on health systems, with the risk of multiple waves that may be implied by the mutagenic potential of the virus, the identification of clinical indicators not only associated with the severity of COVID-19 but also that can strengthen the triage of patients admitted to health facilities have become crucial. This will provide data for the development of patient care protocols in order to preserve their vital prognosis, especially in low-income countries [17].It is in this context that we proposed to explore the role of dyslipidemia in the severity of COVID-19 and the prognosis of patients in the sub-Saharan African context, more precisely in Togo.The general objective of our study was to study the possible role of dyslipidemia in the prognosis of COVID-19. More specifically, the aim of our study was to evaluate the frequency of dyslipidemia in patients with COVID-19 in Togo on the one hand, and on the other hand, the association between dyslipidemia and the prognosis of the disease, i.e., in relation to the clinical severity and the outcome of the patient's hospital stay.
2. Method
2.1. Framework of the Study
- We conducted our study at the Regional Hospital of Lomé Commune (CHR-LC), a reference center for the care of COVID-19 in Togo.
2.2. Type and Period of Study
- This was a single-center descriptive and analytical study that spanned a 15-month period from March 21, 2020 to June 18, 2021.
2.3. Study Population
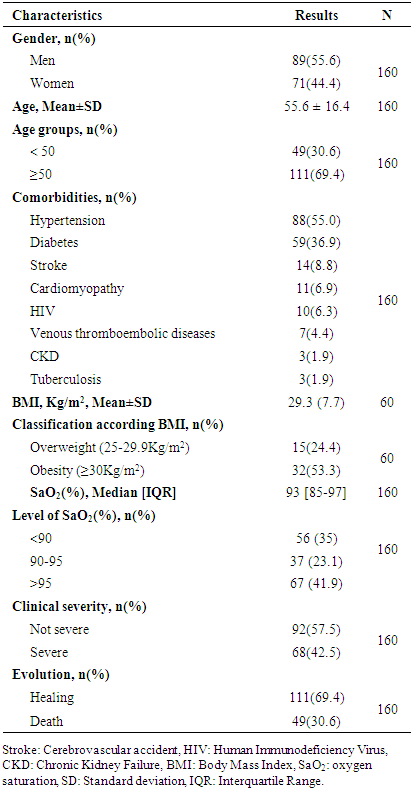
- Our study focused on subjects with COVID-19 admitted to the CHR-LC. The inclusion criterias were patients:- Aged ≥18 years;- having a confirmation of COVID-19 by a PCR test;- with at least the moderate form of COVID-19;- having at least two lipid markers in their medical file.Asymptomatic and mild forms were not included in our study as these clinical forms generally had a favorable prognosis and did not require resuscitative measures.
2.4. Sampling Technique
- The minimum size of our sample was calculated using the Giezendanner formula [18]:n = z2p (1- p) / d2with:n = minimum samplez = confidence level (for a 95% confidence level, z=1.96)p = prevalence of COVID-19d = tolerated margin of error (5%)For better representativeness, we therefore obtained a minimum sample of n= 58 patients. However, the analysis of the files allowed us to include 160 patients in our study.
2.5. Data Collection
- Data were collected from patient hospitalization records using a standardized data collection form. The parameters collected were: (i) Socio-demographic characteristics such as age, gender, education level, occupation, marital status, area of residence, date of admission and discharge; (ii) medical history, such as venous thromboembolic diseases, comorbidities and addictions (alcohol, tobacco, illicit substances); (iii) clinical data including date of onset of symptoms, functional and general signs, degree of clinical severity, and physical findings evaluated on patient admission. (iv) lipid profile, namely total cholesterol, LDL-c, HDL-c, and triglyceride levels; (v) evolution (cure/death). Regarding the forms of clinical severities of COVID, the patients were divided into two groups:- Severe forms which combined severe and critical forms.- The non-severe forms which combined the moderate forms.Dyslipidemia were defined according to the NCEP-ATP III (the National Cholesterol Education Program) criteria:- Total hypercholesterolemia (HCT) defined by a total cholesterol (TC) level ≥ 200mg/dl and- Hypertriglyceridemia (HTG) defined by a triglyceridemia (TG) ≥ 150mg/dl- LDL hypercholesterolemia (HCL) defined by an LDL-Cholesterol level (LDL-c) ≥ 130mg/dl- HDL hypocholesterolemia (HCH) defined by an HDL-Cholesterol (HDL-c) level < 40mg/dl in men and < 50mg/dl in womenThe TC/HDL-c and TG/HDL-c ratio were used to evaluate the atherogenicity of the plasma lipid profile. A TC/HDL-c ratio >5 corresponded to a high risk of atherosclerosis. For TG/HDL-c a threshold ≥4 is considered to be a high risk for cardiovascular incidents.
2.6. Data Processing
- The data were entered in a mask developed with the EPIDATA version 3.1 software and then the statistical analysis of the data was carried out by the IBM SPSS version-20 statistical software. The results were presented in the form of numbers and proportions for the qualitative variables while the quantitative variables were presented in the form of mean/standard deviation or median/interquartile range depending on whether they followed a normal distribution or not. The comparison of qualitative variables was carried out by the Chi 2 test or the Fischer test and the comparison of means and medians was carried out by the Student-t test or the Wilcoxon test respectively. Univariate and multivariate binary logistic regression analysis were used to assess factors associated with the presence of differents profiles of dyslipidemias; the logistic regression models’ results are presented as odds ratios (OR) with the 95% CI. A p-value < 0.05 was considered statistically significant.
2.7. Ethical Considerations
- Patient anonymity was respected and maintained throughout our study.
3. Results
3.1. General Characteristics of the Study Population
- Table 1 shows the main general characteristics of the study population. The male sex was the most represented in our series in 55.6% of cases with a M/F sex ratio of 1.25. The mean age of the study population was 55.6±16.4 years with no significant difference between the mean age of men and women (55.84±15.0 years vs 55.21±17.9 years). The age group of 50 years and over was the most represented in a proportion of 69.4%.
|
3.2. Patients’ Lipid Profile
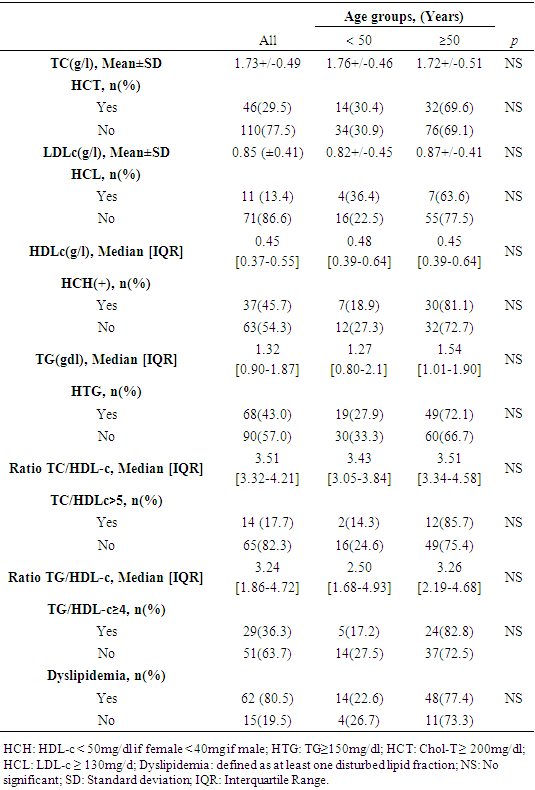
- Table 2 shows the characteristics of the lipid markers explored in our study. HCH was the most represented dyslipidemia in our series with a frequency of 45.7% followed by HTG at 43%. None statistical difference was found between profiles of dyslipidemia and the age of patients.
|
3.3. Dyslipidemia, Clinical Severity of COVID-19 and Evolution
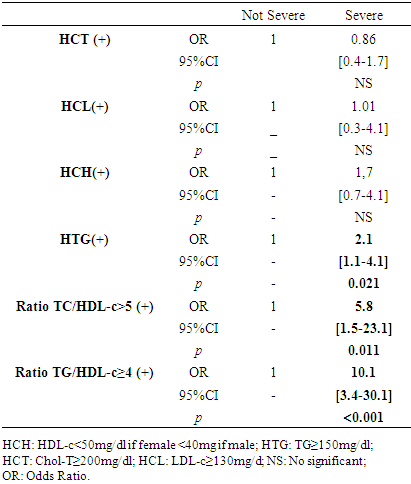
- Tables 3 and 4 present the analysis of the association between dyslipidemia and the clinical severity of COVID-19 as well as the clinical evolution during the patients' hospital stay. Forms of dyslipidemia associated with a greater risk of COVID-19 severity are HTG (OR = 2.1; CI: 1.1 - 4.1; p = 0.021), TG/HDL-c ratio≥4 (OR= 10.1; CI: 3.4-30.1; p<0.001) and a TC/HDL-c ratio≥5 (OR = 5.8; CI: 1.5 - 23.11; p = 0.011).
|
|
4. Discussion
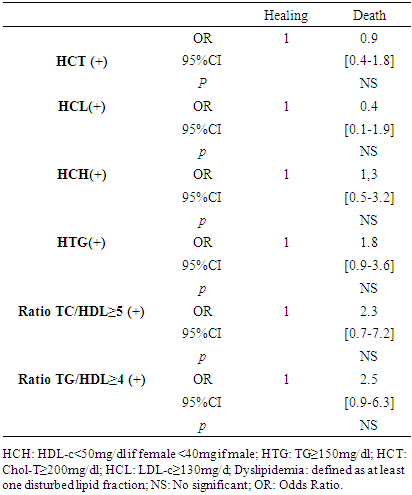
- The frequency of dyslipidemia in our study was high at 80.5%.Studies that have explored dyslipidemias in COVID-19 are mostly systematic reviews and meta-analyses [16,19–21]. The majority of these studies considered the history and comorbidities of patients with COVID-19 to evaluate dyslipidemia, which was defined based on the patients' history. Thus, the frequencies of dyslipidemias in the studies differed considerably. Especially in the Asian studies, the frequency of dyslipidemia was much lower than in the other studies between 1-10% [21–25]. In the USA, dyslipidemia frequencies of 32.5% and 48.7% have been reported [26,27]. In Europe, the frequency of dyslipidemia varied between 28.4% to 65% [28,29]. The fact that dyslipidemias are associated with both environmental and genetic factors may explain these diversities of results. For example, there is a triglyceride paradox that black and Afro-descendant subjects tend to have a low prevalence of HTG despite the presence of high HCH levels [30]. Hyperactivity of lipoprotein lipase and low levels of apolipoprotein CIII compared to the Caucasian subject would be a hypothesis to explain this discrepancy [31].HCH and HTG were forms of dyslipidemia most represented in our study respectively with frequencies of 45.7% and 43%. Our results is in line with the work published by Zaki et al. who in his review, reported that TC, HDL-c and LDL-c levels were systematically lower in the COVID-19 infected group compared to the control group [20]. Our analysis of the association between the different profil of dyslipidemia and the severity of COVID-19 revealed that HTG (OR = 2.1; CI: 1.1 - 4.1; p = 0.021), TG/HDLc ratio≥4 (OR= 10.1; CI: 3.4-30.1; p<0.001) and TC/HDLc ratio (OR = 5.8; CI: 1.5 - 23.11; p = 0.011) are statistically factors associated with severe form of COVID-19. Our results is in line with Alcantara-Alonso et al. who reported an association of TG/HDL-c with COVID-19 severity [32]. Also, Massana et al. et Yoshikawa et al. reported that higher triglyceride levels before infection were associated with more severe forms of COVID-19 [29,33]. If our study did not objectify any profil of dyslipidemia as an independent risk factor associated with the death of patients with COVID-19, Wen Dai et al. have reported an association between HTG and mortality during COVID-19 [34] and Peng et al. an significant association of TG/HDL-c with the mortality due to COVID-19. The prognosis of COVID-19 involves an overriding inflammatory component with the cytokine storm. Inflammation predictors strongly related to the cytokine storm have been identified during COVID-19, namely C-reactive protein (CRP), serum ferritin, lactate dehydrogenase (LDH) [35]. Zhong et al. showed these cytokine storm’s predictors were significantly correlated with elevated TG levels in COVID-19 patients [36]. Specially for LDH, it has been reported a positive association been elevated LDH and all-cause mortality in individuals with metabolic syndrome [37]. HTG which is a component of the definition of metabolic syndrome is also well knowned as an activator of the NLRP3 inflammasome which triggers the secretion of adipokines and cytokines that induce systemic inflammatory states favorable to viral infections such as COVID-19 or HIV [38]. HCH and HTG are two forms of dyslipidemias that fit into the definition of metabolic syndrome with excess weight, high blood pressure and hyperglycemia. Our study identified excess weight in 77.7% of cases, hypertension in 55% and diabetes in 36.9%, which makes them the main pre-existing morbid conditions in the patients in our study before their episode of COVID-19. With a frequency of dyslipidemia of 80.5% that we objectified in our study, it is not excluded that many patients had a metabolic syndrome which, because of the chronic inflammation, oxydative stress and endothelial dysfunction that result from it, could be linked to the severity of COVID-19. Hence the hypothesis that dyslipidemia could therefore play an indirect role in the severity of COVID-19. This hypothesis is supported by the fact that our study identified TG/HDLc ratio≥4 (OR= 10.1; CI: 3.4-30.1; p<0.001), TC/HDL-c ratio≥5 (OR = 5.8; CI: 1.5 - 23.11; p = 0.011), and HTG (OR = 2.1; CI: 1.1 - 4.1; p = 0.021), as factors associated with the clinical severity of COVID-19. This indicates that the impact of dyslipidemia on vessel permeability could secondarily result in severe form of COVID-19 and poor prognosis. Not only can this alteration of capillary permeability be linked to atherosclerosis in connection with the TG/HDL-c ratio but also because of the inhibition of lipoprotein lipase (LPL) induced by inflammation and infection [39]. LPL is attached to the surface of the capillary endothelial lumen by a protein hepran sulphate peptidoglycans (HSPG) in order to facilitate its hydrolysis action on triglycerides and therefore their clearance [39]. In cases of severe infection, HSPG is eliminated from the endothelium leading to LPL dysfunction and therefore to HTG [40]. HTG will therefore trigger or even contribute to exacerbated systemic inflammation with the links it maintains as a component of the metabolic syndrome, i.e. through the correlation it has with the predictors of systemic inflammation.Hence, the interest that the evaluation of TG, TG/HDL-c and the TC/HDL-c ratio could be considered as indicators for evaluating the potential involvement of dyslipidemia in the severity of COVID-19. It should be noted that our study, like most retrospective studies, was confronted with the lack of completeness of the data, particularly of the lipid profile in certain patients. Lipid profile realization was not routine in patients with COVID-19 at the beginning of the pandemic, especially in those with mild severity. As a multidisciplinary team was set up at the instigation of the health authorities to deal with the influx of patients, the composition of the standard assessment for each new admission was expanded. This is the reason why the dosages of the LDL-c and HDL-c fractions were missing in some files. It should also be noted that data on dyslipidemia were collected from test results available in the patients' files. However, these analyses were performed in different laboratories (public and private), especially when patients were referred from another health care facility to the CHR-LC with all the examinations. Under these conditions, it is impossible to guarantee the standardization of the techniques used by each laboratory in the dosage of the different lipid fractions, which constitutes a limitation to our study. If, in view of the above and its monocentric nature, our study cannot be extrapolated to all patients with COVID-19 in Togo, it is nevertheless of interest because, to our knowledge, it is one of the first studies in Togo to explore the involvement of dyslipidemia in the severity of COVID-19.
5. Conclusions
- Finally, we note that dyslipidemia is quite frequent in patients with COVID-19 in Togo and is found in 08 cases out of 10 (80.5%). Obesity, hypertension and diabetes as the main comorbidities found in patients with HCH and HTG as the major forms of dyslipidemia highlighted draws a constellation evoking the metabolic syndrome, which would contribute indirectly to the severity of the disease and a poor prognosis.
Financial Disclosure
- This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
- Data can be acquired from corresponding author on reasonable request.
Declaration of Competing Interest
- The authors declare no conflict of interests for this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML