-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2021; 10(2): 25-30
doi:10.5923/j.ijim.20211002.01
Received: May 8, 2021; Accepted: May 25, 2021; Published: May 28, 2021

Determinants of the Time of Occurrence of Deaths During Covid-19 in Togo: A Report on 67 Cases
Abdou Razak Moukaila1, 2, Lidaw Déassoua Bawe2, 3, Awereou Kotosso2, 3, Yawovi Mawufemo Tsevi2, 4, Gnimdou Tchamdja2, 5, Archad Bozinabo Nekere6, Agbeko Kodjo Djagadou1, Komi Séraphin Adjoh7, Majesté Ihou Wateba3, Awalou Mohaman Djibril1, 6
1Department of Internal Medicine, Sylvanus Olympio University Hospital, University of Lomé
2Medical Team, Lomé-Commune Regional Hospital
3Department of Infectious and Tropical Diseases, Sylvanus Olympio University Hospital, University of Lomé
4Department of Nephrology and Hemodialysis, Sylvanus Olympio University Hospital, University of Lomé
5Department of Intensive Care, Sylvanus Olympio University Hospital, University of Lomé
6National Coordination of Management Response against COVID-19
7Department of Pneumology and Phthisiology, Sylvanus Olympio University Hospital, University of Lomé
Correspondence to: Abdou Razak Moukaila, Department of Internal Medicine, Sylvanus Olympio University Hospital, University of Lomé.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: The COVID-19 pandemic has resulted in millions of deaths around the world with a heavy impact on social and health systems. The objective of this study was to analyze not only the profile of cases of deaths that have occurred since the advent of the pandemic in Togo, but also the determinants of their time of occurrence. Method: This was a cross-sectional study in which the Lomé-Commune Regional Hospital, a COVID-19 care center, served as the framework for our study. The study population consisted of all patients admitted for COVID-19 confirmed by a positive PCR test for SARS-COV-2, aged 18 years and over and who died during their hospital stay. Results: Sixty-seven patients were included. Males were most represented in 62.7% of cases. Almost six out of 10 patients were over 50 years old. The two main comorbidities found in the patients were hypertension (44.8%) and diabetes (28.4%). The majority of patients had the severe form of COVID-19 in 46.3% of cases. Slightly less than half of the patients (49.3%) died within five days of admission. Degree of glycemia elevation (p<0.01), AST elevation (p = 0.012), and ALT elevation (p<0.01) levels were statistically associated with time of occurrence of deaths. Conclusion: Glycemia and liver enzyme disturbances (AST and ALT) can be used as prognostic predictors in the sorting of patients admitted to hospital for COVID-19 and thus plan the implementation of adequate therapies to safeguard their vital prognosis.
Keywords: COVID-19, Death, Togo, Glycemia, ALT, AST, SARS-COV-2
Cite this paper: Abdou Razak Moukaila, Lidaw Déassoua Bawe, Awereou Kotosso, Yawovi Mawufemo Tsevi, Gnimdou Tchamdja, Archad Bozinabo Nekere, Agbeko Kodjo Djagadou, Komi Séraphin Adjoh, Majesté Ihou Wateba, Awalou Mohaman Djibril, Determinants of the Time of Occurrence of Deaths During Covid-19 in Togo: A Report on 67 Cases, International Journal of Internal Medicine, Vol. 10 No. 2, 2021, pp. 25-30. doi: 10.5923/j.ijim.20211002.01.
Article Outline
1. Introduction
- Since the first case reported in December 2019 in Wuhan, China, the rapid spread of the virus around the world led the World Health Organization to declare COVID-19 as a pandemic on March 11, 2020. COVID-19 has caused nearly 2,907,944 deaths worldwide and has had a significant impact on the health systems of countries that were unprepared for such a deluge of patients requiring intensive care measures. In addition to the impact on health systems, the economic and social consequences of border closures, confinement measures thus impacting the free movement of goods and people. The year 2021 begins with the deployment of vaccines but also and above all with the prospect that from now on, humanity will have to cohabit with COVID-19 in view of the mutagenic potential of the virus.The worst scenarios had been imagined for Africa, the latter accounted for approximatively 2.8% of deaths worldwide as of 02/15/2021 [1]. The extreme anticipation with which African authorities reacted to contain the spread of the virus by closing borders and adopting confinement measures, the youth of the population, and climatic predisposition [2] are among the hypotheses put forward to explain this situation, which thwarted the darkest prognoses for the continent. However, we must remain vigilant insofar as, sub-Saharan Africa where Togo is located globally has a fragile health system and a population with low purchasing power, the vast majority of which does not have universal social coverage.Togo, a West African country located between Benin in the East, Ghana in the West and Burkina Faso in the North, has reported 11,814 cases to date, including 116 deaths [1]. Faced with the evolution of COVID-19 towards an increasingly community-based mode of transmission, it is necessary, in order to ensure proper management of cases which will be detected and admitted in hospital to draw the lessons from previously recorded deaths. That will enable clinicians to make rapid and appropriate decisions to preserve the vital prognosis of patients.We propose through this work to describe the epidemiological characteristics of deaths due to COVID-19 in Togo and examine the factors associated with the time of occurrence of death.
2. Method
2.1. Presentation of the Study Site
- Our study took place at the Lomé-Commune Regional Hospital, a reference center for the management of COVID-19 cases.
2.2. Type and Period of Study
- This was a cross-sectional study covering the period from March 06, 2020 to December 31, 2020, i.e. a period of 09 months.
2.3. Study Population
- The study population consisted of all patients admitted for COVID-19 confirmed by a positive PCR test for SARS-COV-2, aged 18 years and over and who died during their hospital stay in the care center. In addition to the socio-demographic information, the patient’s medical record had to include, the date of admission and the date of death, any comorbidities and data relating to venous glycemia, serum creatinine and transaminases measurements carried out at the patient’s admission.
2.4. Data Sources and Collection
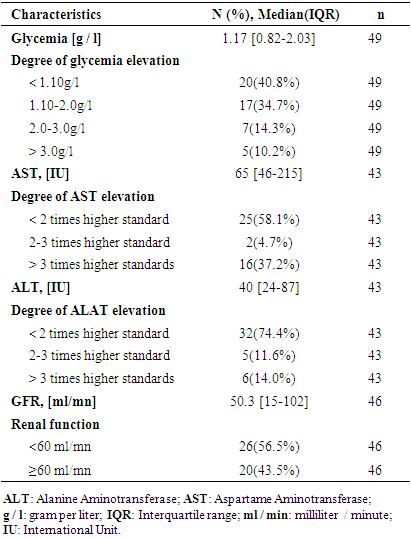
- We extracted deidentified data from the COVID-19 hospital registry and our variables of interest were: (i) socio-demographic data, age, gender; occupation, marital status, level of education (ii) co-morbidities, namely hypertension (HTA), diabetes, HIV, heart disease, cancer, bronchopneumonia, renal failure, liver disease; (iii) clinical data relating to the patient's hospital stay, i.e. date of admission and the date of death, from which we calculated the length of hospital stay, the WHO clinical classification of Covid-19; (iiii) venous blood glucose on admission, creatinine clearance, Alanine Amino transferase (ALT) and Aspartame Amino Transferase (AST) transaminase assay. The MDRD formula was used to evaluate creatinine clearance. Patients were further divided according to their glycemia level on admission into four groups: < 1.10g/l; 1.10-2g/l; 2-3g/l; > 3g/l. Acute liver injury in our study was defined as ALT elevation greater than twice the upper limit or a conjugated increase in AST. For our study, we considered the upper limit of AST and ALT levels to be 40 IU/l. A renal clearance < 60ml/min was considered disturbed while clearance ≥60ml/min was the translation of undisturbed renal function.
2.5. Statistical Analysis of Data
- All data were entered into a data entry mask developed in Epi data version 3.1 software and then analyzed using IBM SPSS Statistics 20 statistical software.Descriptive analysis included medians and interquartile range for quantitative variables.Categorical data were expressed as percentages. The chi-square test was used to compare proportions. A p-value < 5% was considered statistically significant.
2.6. Ethical Consideration
- Permission was granted from the COVID-19 response coordinator and the patient confidentiality was respected throughout the study.
3. Results
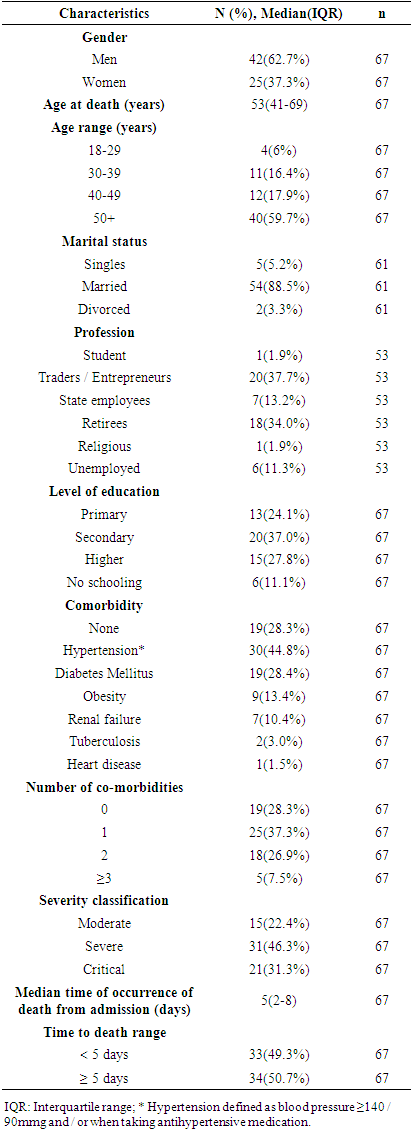
- During our study period, 68 deaths, including an infant under one year old were recorded out of a total of 3576 confirmed cases corresponding to a case fatality rate of 1.9% [3]. Sixty-seven patients were therefore selected for our study.Table 1 shows the socio-demographic characteristics of the deceased patients included in our analysis. Males were most represented in 62.7% of cases with an M/F sex ratio of 1.68. Almost six out of 10 patients were over 50 and slightly less than nine out of 10 were married. The most represented socio-professional class was that of traders or independent entrepreneurs. The three main comorbidities found in the patients were hypertension (44.8%), diabetes (28.4%) and obesity (13.4%). In terms of severity, the majority of patients on admission were classified as severe stage in 46.3% of cases, followed by critical stage in 31.3% of cases. In almost half of the cases (49.3%), death occurred in less than five days after admission.
|
|
|
4. Discussion
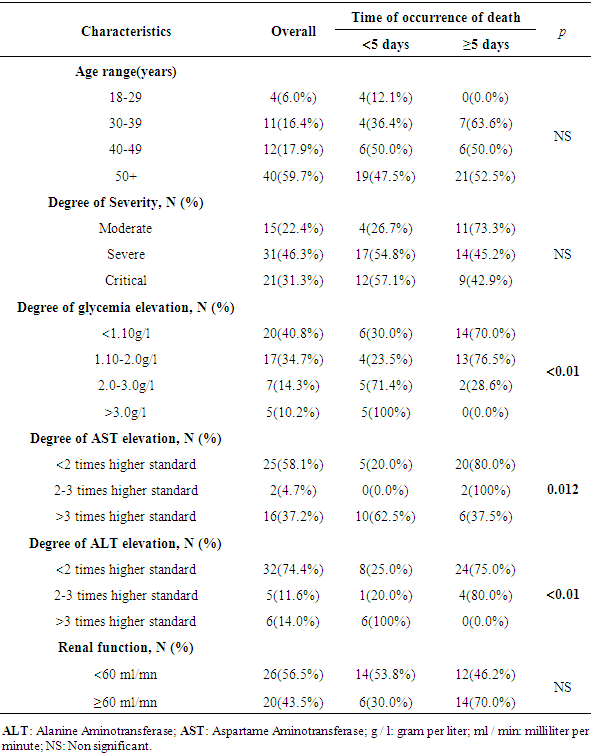
- The objective of our study was to describe the epidemioclinical profile of deaths due to COVID-19 in Togo and to analyze the factors associated with the time of occurrence of death.Our study found that males were predominant in 62.7% of cases with an M/F sex ratio of 1.68. Male predominance in COVID-19 related deaths has been reported by many studies in very similar proportions as was the case of Wang et al., Zang et al. and Yao et al. who reported 66.2%, 65.9% and 63.9% respectively [4–6]. The median age of our study population was 53 years (IQR: 41-69). This result is below the median age of deaths reported in China and the United States respectively 72.5 years (IQR: 65-80) and 78 years (IQR: 67-87) [5,7]. Moreover, the proportion of patients over 50 years old (59.7%) in our study was lower than that of the same age group reported in China and the United States, where the proportion of subjects over 50 years of age exceeded the 90% [5–7]. This difference in ages is linked to the age pyramid of sub-Saharan African countries which have a broader base reflecting a younger population compared to developed countries. Seventy-one point seven percent of patients in our study had at least one comorbidity.This result is consistent in the literature, where patients presented in more than 70% of cases at least one comorbidity, the first three of which were represented by hypertension, diabetes and cardiomyopathies, as revealed in our study [4–6,8]. The median time from hospital admission to death was 5 days (IQR: 2-8) in our study. This result is similar to those reported in the United States, i.e. 5 days (IQR: 3-8) [7], Italy 6 days (IQR: 3-10) [8] and Indonesia 6 days (IQR: 2-11) [9]. We found a statistically significant association between the time of occurrence of death from admission and the rise in glycemia level. Our result is in line with studies that have reported that hyperglycemia was a predictor of poor prognosis in hospitalized COVID-19 patients [10] or even associated with a particularly high mortality rate in patients with uncontrolled hyperglycemia [11].Even more, Carrasco-Sanchez et al. reported that hyperglycemia on admission was a strong predictor of any cause of mortality in patients hospitalized for non-critical COVID-19 regardless of whether or not there was an antecedent of diabetes [12]. The hyperglycemia of patients with COVID-19 finds its source in the direct attack of the endocrine pancreas that SARS-COV-2 is likely to cause leading to insulinopenia [13], but also in the cytokine tornado which by the proinflammatory cytokines released will cause insulin resistance and then insulinopenia secondary to glucotoxicity on Langherans beta cells [14,15]. Secondary hyperglycemia will be the vector of a prothrombotic state due to oxidative stress which can lead to multivisceral damage [16]. Also, it should be noted that the binding of SARS-COV-2 to its ACE2 receptor requires the glycosylation of the latter. As a result, a state of hyperglycemia will lead to hyperglycosylation of ACE2 receptors, favoring infection of the organism by SARS-COV-2 and thus aggravation of the disease [17]. It therefore appears from the above, the interest to ensure an early and close control of glycemia from the early stage of the disease by giving priority to the administration of ordinary insulin to COVID-19 patients admitted to hospital [18].Our study has showed a significative association between the time of occurrence of death and the liver enzymes levels AST and ALT. No antecedent of liver disease was reported in patients' records included in our study. However, it is impossible for us to exclude the possibility of a pre-existing chronic hepatopathy due to various causes such as diabetes, drug or ethyl intoxication, unrevealed steatosis in some patients, which could explain the elevation of liver enzymes before the occurrence of COVID-19. In the hypothesis that the existence of an unknown pre-existing liver disease would have impacted on the prognosis of the patients, it has been reported that the existence of a chronic liver disease was not associated with the prognosis of COVID-19 patients [19] and that the frequencies of the underlying chronic liver disease were not statistically different depending on whether the patients were classified as severe or not [20]. However, in line with our results, it has been reported that patients with liver disease were more numerous among patients classified as severe than moderate with a significant risk of poor prognosis or even death [19,21–23]. Specifically, a disturbed AST level on admission has been reported as an independent predictor of severity and mortality due to COVID-19 [24,25]. ACE2 receptors are more expressed in the common bile duct than in hepatocytes [26]. However, cholangiocytes play an important role in the protection and regeneration of the liver [27]. SARS-Cov2 would lead to direct destruction of liver tissue by direct action on cholangiocytes but also through the inflammatory syndrome [28–30]. Hepatic damage leads to an increased in the expression of ACE2 receptors in the hepatocytes, which subsequently worsened liver infection [31].Our study has limitations that are worth noting. First, its retrospective and monocentric nature not only makes it possible to establish a causal link between the studied parameters but also a generalization of the results to the entire population affected by COVID-19 in Togo. Then, the insufficiency and absence of certain data made it impossible to explore other factors described in the literature as associated with death during COVID-19 such as C-reactive protein, D-dimers, lactate dehydrogenase, procalcitonin. Nevertheless, our study has the merit of providing clues in the analysis of COVID-19 cases admitted to hospital, allowing clinicians to better sort and takes care of patients.
5. Conclusions
- Hyperglycemia and liver enzyme disturbances (AST and ALT) detected at admission of COVID-19 hospitalized patients are correlated with an early time of occurrence of death. These biological parameters can therefore be useful in the sorting of patients and in planning the implementation of an adequate therapeutic protocol to preserve the patient's vital prognosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML