-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2021; 10(1): 18-23
doi:10.5923/j.ijim.20211001.03
Received: Feb. 23, 2021; Accepted: Mar. 17, 2021; Published: Apr. 15, 2021

Complete Resolved Right Atrial Lymphoma after Chemotherapy in a Patient Diagnosed with Classical Nodular Sclerosis Hodgkin Lymphoma
Mohamed Wael1, Mariam Al-Hammadi1, Daher Alarab1, Salwa Aly1, Hosameldin Mohamad1, Walid Assar1, 2
1Internal Medicine and Radiology Departments, King Hamad University Hospital, Egypt
2Department of Cardiology, Faculty of Medicine, AL-Azhar University, Egypt
Correspondence to: Walid Assar, Internal Medicine and Radiology Departments, King Hamad University Hospital, Egypt.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hodgkin lymphoma counts for 15 to 25% of all lymphomas. Cardiac tumors are divided into primary and secondary tumors. Cardiac Lymphomas whether primary or secondary are rare. The most common imaging modality used to diagnose cardiac tumors is Echocardiography. Trans esophageal Echocardiography is superior to Transthoracic Echocardiography with higher sensitivity and specificity. Positron-emission tomography (PET)/ Computed Tomography scan and cardiac Magnetic Resonance Imaging (MRI) are other modalities used for diagnosis. Chemotherapy is currently the main treatment option for Lymphomas. We report a case of a 31-year-old-male who presented with abdominal pain, weight loss and intermittent vomiting and was diagnosed to have Hodgkin’s Lymphoma with cardiac involvement. Patient received chemotherapy and showed complete resolution. In this case report, we compare the different imaging modalities including CT scanning, Transthoracic Echocardiography, Trans-esophageal Echocardiography, and the PET/CT Scan that was done for the patient before and after treatment.
Keywords: Hodgkin lymphoma, Cardiac tumors, Trans-Thoracic echocardiogram, Positron-emission tomography (PET) scan, Trans-esophageal echocardiogram
Cite this paper: Mohamed Wael, Mariam Al-Hammadi, Daher Alarab, Salwa Aly, Hosameldin Mohamad, Walid Assar, Complete Resolved Right Atrial Lymphoma after Chemotherapy in a Patient Diagnosed with Classical Nodular Sclerosis Hodgkin Lymphoma, International Journal of Internal Medicine, Vol. 10 No. 1, 2021, pp. 18-23. doi: 10.5923/j.ijim.20211001.03.
1. Introduction
- Hodgkin lymphomas are lymphoid neoplasms in which malignant Reed-Sternberg cells are admixed with a heterogeneous population of non-neoplastic inflammatory cells [1]. Classical Hodgkin Lymphoma is the most common type and accounts for 15% to 25% of all lymphomas [2]. The five-year survival rate of classical Hodgkin Lymphoma in those diagnosed at ages 20-64 can reach up to 89.8% with treatment [3]. Cardiac tumors can be classified into primary and secondary (metastatic) tumors and are rare disorders. At the Royal Brompton Hospital, London, United Kingdom, over the last 20 years, a total of 94 patients with a histological diagnosis of primary cardiac and pericardial tumors were identified. As observed in other series, the majority (n = 67; 71.3%) of cases were benign, with myxoma being the most common histologic type accounting for 27 cases. Primary malignant tumors (n = 27; 28.7%) included unclassified sarcoma (n = 11), leiomyosarcoma (n = 5) and lymphoma (n = 4) as the most common histologic types [4].In a study in Hong Kong, which looked at more than 12,000 autopsies, the incidence for primary and secondary heart tumors was 0.056% and 1.23% respectively [5]. In the same study, lymphomas were found in 11.9% of males and in 17% of females with secondary cardiac tumors. [5] Approximately, 1.8% of Primary cardiac tumors are thought to be secondary to Lymphomas [6]. However, the incidence of Hodgkin’s Lymphoma presenting as secondary cardiac tumor is unknown.Symptoms secondary to Cardiac tumors may occur through one of four mechanisms. First, the tumor might obstruct the blood flow through the heart or interfere with valve function. Secondly, local Invasion can lead to arrhythmias or pericardial effusion with or without tamponade. Thirdly, embolization can occur from tumors on the left side of the heart and lead to systemic manifestations and finally these tumors might cause constitutional symptoms. [7] Most patients present with symptoms of congestive heart failure or superior vena cava obstruction; after which, the second most common presenting symptom is embolization [8]. Cardiac tumors can also present with various types of arrhythmias according to tumor type and location [9].On the other hand, tumors can also be present without causing any symptoms and become evident by incidental findings, especially today with the increased use of noninvasive imaging techniques.The most common imaging used to diagnose Cardiac Tumors is Echocardiography. Trans esophageal Echocardiography is superior to Transthoracic Echo with higher sensitivity and specificity [10]. Other modalities include cardiac Magnetic Resonance Imaging (MRI) and Cardiac Computed Tomography (CT) scanning [11]. Cardiac MRI is considered the most accurate in diagnosing cardiac tumors and can help differentiate benign from malignant tumors [12]. Positron Emission Tomography (PET)/CT scan is another modality that can visualize cardiac tumors and was reported in a number of case reports [13], [14]. The disadvantage with PET/CT scan is that it cannot differentiate a clot from a tumor as both show increased Fluorodeoxyglucose (FDG) uptake. We report a 31-year-old male diagnosed with Hodgkin’s Lymphoma with cardiac involvement, the different imaging modalities used and the response to treatment.
2. Case Report
- A 31-year-old male patient presented to the Emergency Department with 1-day history of severe, continuous, generalized abdominal pain associated with multiple attacks of vomiting which was accompanied with constipation of 3 days duration. X-ray abdomen also showed air fluid levels. On further history taking, the patient gave a history of unintentional weight loss of around 20 kilograms over the last 6 months with a complain of night sweats as well. Patient was also having epigastric pain for the same period associated with intermittent dysphagia to solids mainly but not to liquids. The patient did not have any chest pain, palpitations, arrhythmias, evidence of heart failure or any other cardiac complains. On examination, he was vitally stable and afebrile. He had generalized tenderness in the abdomen. Cardiovascular examination was unremarkable. Patient was also found to have enlarged cervical Lymph nodes on examination. His White Blood Cell count was 14,000 x10^9/L. Otherwise Hemoglobin, platelets, renal function, coagulation and liver function were unremarkable. CT Abdomen was done for the patient which did not reveal any obstruction but revealed distal jejunal loops and proximal ileal loops wall thickening. Also found in the peritoneal/retro-peritoneal region was soft tissue small masses suggesting enlarged Lymph nodes seen in relation to left adrenal gland vicinity, one shows cystic changes within (2cm). Chest cuts (figure 1) revealed right atrial soft tissue mass lesion measures 4x2.8 cm in diameter. Also diffuse pericardial soft tissue thickening was noted.
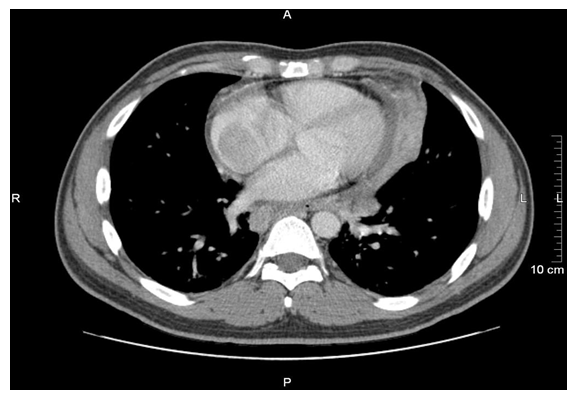 | Figure 1. CT chest before the treatment. There is right atrial soft tissue mass lesion measures 4x2.8 cm in diameter. There is also diffuse pericardial soft tissue thickening |
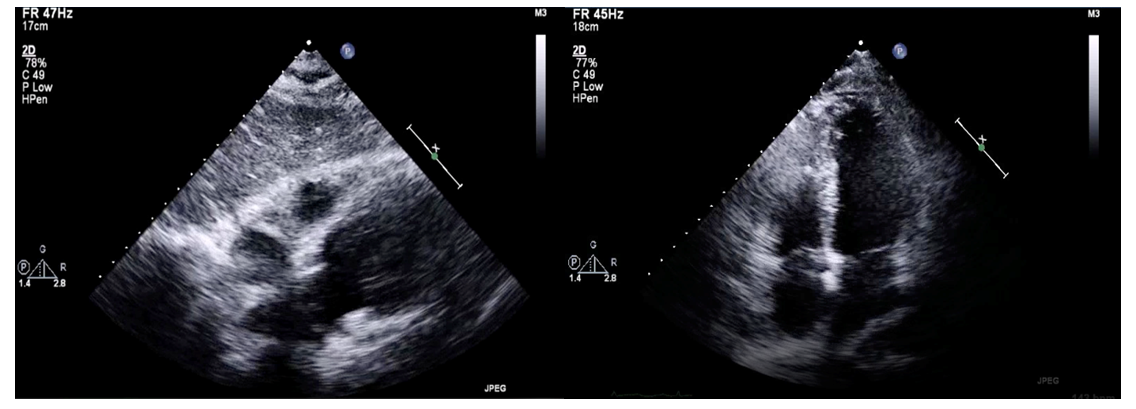 | Figure 2. TTE done which was unremarkable |
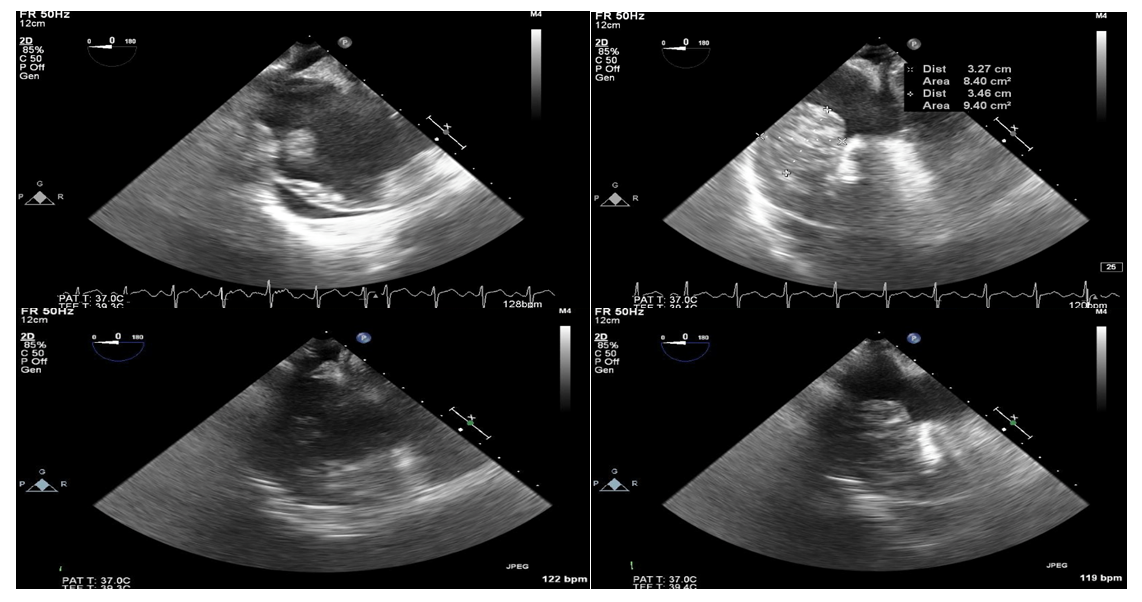 | Figure 3. TEE done which showed right atrial and pericardial infiltration |
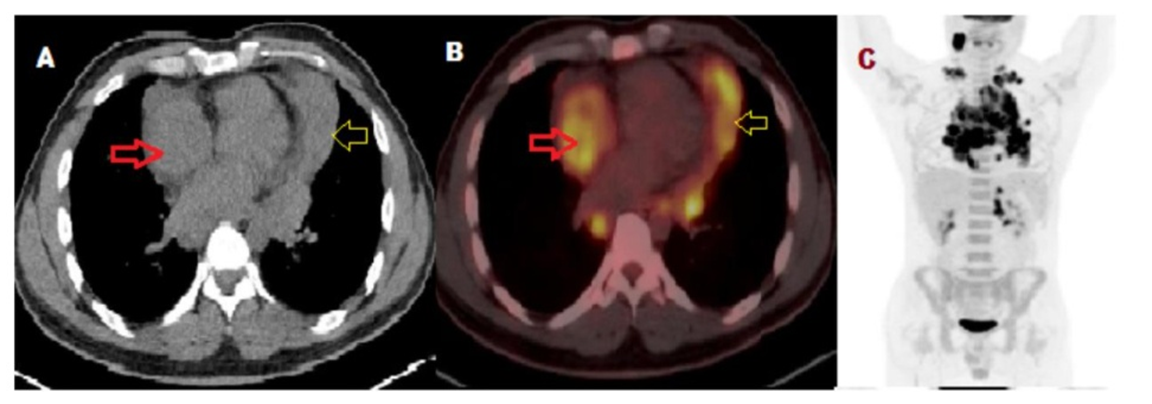
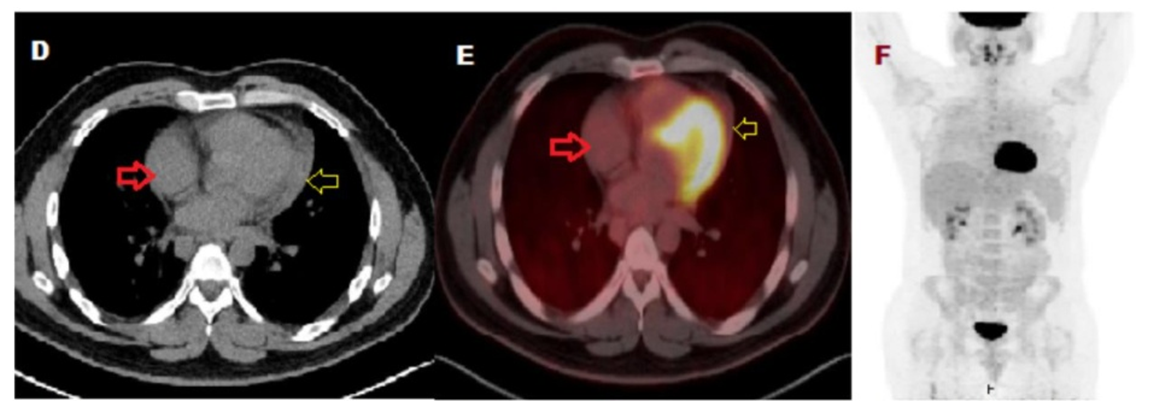 | Figure 5. D: axial CT, E: Fused axial PET/CT and F: maximum intensity projection for a whole-body of FDG-PET imaging. PET scan showing complete resolution of the above noted cardiac masses |
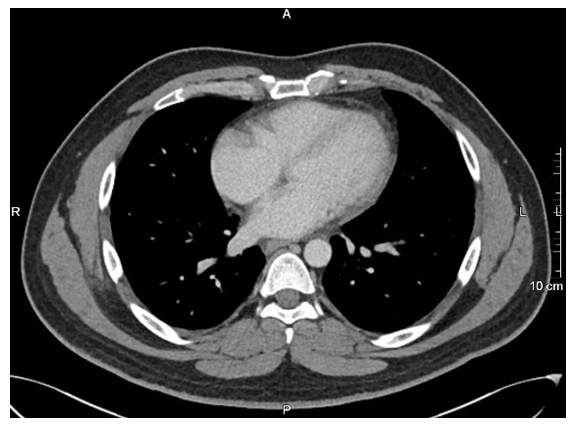 | Figure 6. CT chest after the treatment. There is complete resolution of the right atrial mass and pericardial thickening |
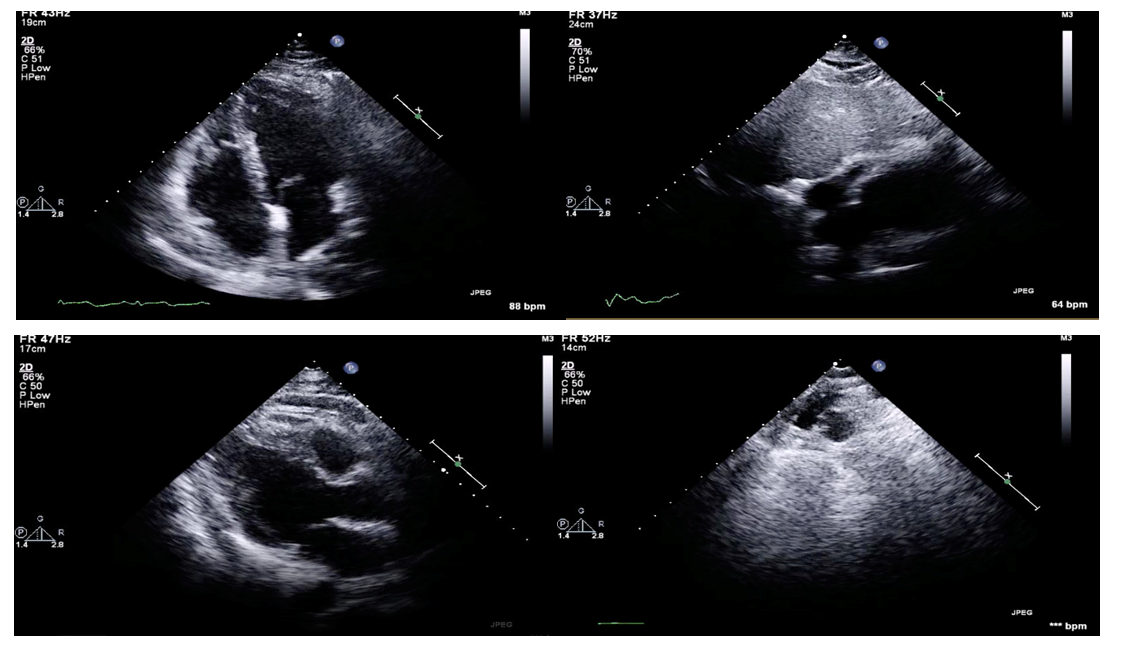 | Figure 7. Follow up TTE showing no cardiac lesions |
3. Discussion
- Primary cardiac lymphoma is exceedingly rare and reflects 1% of all cardiac tumors. However, secondary cardiac involvement from disseminated lymphomas is considered more common and can be seen up to 20% in patients with lymphoma [15]. Right Atrium and right ventricle are the commonest site of cardiac lymphoma although it can spread to the pericardium and different focal areas, yet heart valves are spared [16]. Cardiac involvement can arise in different routes; can occur through direct extension from mediastinal lymphoma, hematogenous spread, and retrograde lymphatic spread [17].Secondary cardiac involvement can present clinically based on the location of the tumor in the heart. Left-sided tumors usually present with Mitral valve disease, heart failure, and secondary pulmonary hypertension; patients present with dyspnea, orthopnea, paroxysmal nocturnal dyspnea, or pulmonary edema. Moreover, left-side tumors can cause embolization and subsequent neurological complication. Right side tumors present with tricuspid stenosis, right-side heart failure, or restrictive cardiomyopathy; patients present with peripheral edema, hepatomegaly, ascites, and shortness of breath (18). More commonly cardiac lymphoma can be detected incidentally on imaging. Our patient did not have any of the above manifestations.Trans-esophageal echocardiogram (TEE) is the first-line investigation for disseminated lymphoma with cardiac involvement, with sensitivity around 60% [19]. A trial conducted between 1989 till 1993 to compare trans-esophageal echocardiogram (TEE) Vs. trans-thoracic echocardiogram (TTE) on 23 patients found that TEE detected three tumors that were not detected by TTE. Besides, TEE showed additional findings were not seen by TTE. In conclusion, TEE was superior to TTE in detecting cardiac lymphoma especially right heart involvement [20]. This goes in line with our patient was the TTE failed to demonstrate any cardiac lesion. 18-Fluorodeoxyglucose positron-emission tomography (PET) CT provides better anatomical and functional imaging then PET scan alone. It can be used for disseminated lymphoma staging and is also the modality of choice for early diagnosis and monitoring of treatment response [21]. Cardiac magnetic resonance imaging (CMRI) can be used to evaluate cardiac mass character, myocardium, and pericardial involvement. CMRI has a higher value to detect tumor infiltration in comparison to tissue catheterization [22]. Combined PET/MRI modality is the new approach to diagnosis cardiac tumor however due to limited availability remain under clinical trial [23].Treatment of cardiac lymphoma can vary from surgery to chemotherapy alone or combined with radiotherapy. A study conducted in San Raffaele in Milan; Italy observed different management approaches for 48 patients with primary cardiac lymphoma. Eight patients underwent subtotal surgery where one patient only underwent a cardiac transplant and given the postoperative complications and tumor recurrence the patient passed away. On the other hand, 26 patients received chemotherapy mainly CHOP regimen. Radiotherapy was given to 8 patients with or without chemotherapy. The outcome of the study was that there was longer survival with chemotherapy in comparison to radiotherapy or surgery [24].The primary choice of chemotherapy in Nodular sclerosis Hodgkin’s Lymphoma is ABVD protocol in patients with advanced stages. Different randomized trials were conducted comparing ABVD with different regimens such as MOPP (Mechlorethamine, Vincristine, Procarbazine, and Prednisone), Stanford V Regimen, and BEACOPP protocol. In general, ABVD showed better outcome with well-tolerated side effects in comparison to other protocols [25] [26] [27]. In this case, we presented a young male who was diagnosed with Hodgkin’s Lymphoma with cardiac involvement. The interesting thing about this patient was the fact that he did not have any of the symptoms or arrhythmias that such patients might have. Furthermore, the patient showed a complete response to chemotherapy as evidenced by the follow-up PET/CT Scan and CT scans. Cardiac MRI is unfortunately not available in our center and therefore was not used. The patient is currently doing well and has been following with Hematology for 1 year now since completion of chemotherapy.
4. Conclusions
- Cardiac lymphomas are rare and usually detected incidentally on imaging. This case illustrated the use of a number of modalities including Transthoracic echo (TTE), TEE, CT and PET Scan in diagnosing cardiac tumors and demonstrating the response to chemotherapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML