-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2021; 10(1): 6-17
doi:10.5923/j.ijim.20211001.02
Received: Jan. 31, 2021; Accepted: Feb. 15, 2021; Published: Feb. 22, 2021

Vitamin D Testing and Replacement Patterns Among Adults in a Large Medical Centre
Melissa My Chow1, Calvin Yc Chan2, Yong Zhi Chen2, Yan Zhi Tan1, Giat Yeng Khee1, Mcvin Hh Cheen1, Cheryl Yl Lim1, Wan Chee Ong1, Yan Qin3, Matthew Zw Tan4
1Department of Pharmacy, Singapore General Hospital, Singapore
2Department of Pharmacy, Faculty of Science, National University of Singapore
3Department of Internal Medicine, Singapore General Hospital
4Department of Endocrinology, Singapore General Hospital
Correspondence to: Melissa My Chow, Department of Pharmacy, Singapore General Hospital, Singapore.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Increased 25-hydroxyvitamin D (25OHD) testing, driven by studies linking vitamin D (VD) deficiency with many diseases has been challenged due to cost and uncertain health benefits. Insight to current practices in VD testing and replacement can inform development of strategies to ensure judicious testing. Objectives: To assess trends in 25OHD testing and appropriateness, VD status change, VD regimens prescribed, and factors associated with reaching sufficient levels at retest. Design: Single center retrospective large data analysis study in Singapore General Hospital. Subjects: Adults with 25OHD assays performed from 2011 to 2016. Main measures: Data (25OHD tests, patient demographics, co-morbidities and medications) were electronically extracted. Linear regression and chi-square test were used to compare annual change for continuous and categorical variables respectively. McNemar test was used to compare VD status change at retest between adults with low (25OHD <30ng/mL) and sufficient (25OHD >30ng/mL) baseline levels. Multivariate logistic regression was used to identify factors associated with achieving sufficient levels. Key Results: Total of 25,502 25OHD tests were performed in 15,605 adults. Annual tests increased 3.4 fold from 2,125 to 7,236 tests in 2012 and 2016 (p<0.05). Almost 81% of adults had risk conditions, which included chronic kidney disease (57.6%) and fractures (29.6%). Among 4,120 adults with repeated tests, VD status didn’t change significantly between adults with baseline low and sufficient levels (p=0.26). Prescribed VD regimens included VD products at doses <800units/day (42.1%) and cholecalciferol 1,000units/day (32.1%). Factors with higher odds in achieving retest sufficient levels were adults with no risk conditions (OR 1.29, CI 1.03-1.62, p=0.03) and high VD doses (>1,000units/day) (OR 1.23, CI 1.003-1.51, p=0.05). Conclusion: There was an uptrend in 25OHD tests over time. While adults were aptly tested, VD status didn’t improve with repeated testing. High VD doses and no risk conditions were associated with achieving sufficient levels.
Keywords: Vitamin D testing, Vitamin D replacement therapy, 25-hydroxyvitamin D
Cite this paper: Melissa My Chow, Calvin Yc Chan, Yong Zhi Chen, Yan Zhi Tan, Giat Yeng Khee, Mcvin Hh Cheen, Cheryl Yl Lim, Wan Chee Ong, Yan Qin, Matthew Zw Tan, Vitamin D Testing and Replacement Patterns Among Adults in a Large Medical Centre, International Journal of Internal Medicine, Vol. 10 No. 1, 2021, pp. 6-17. doi: 10.5923/j.ijim.20211001.02.
1. Introduction
- Emerging evidence suggesting vitamin D (VD) benefits extending beyond musculoskeletal health [1] has led to increased VD testing among adults in many developed countries. VD deficiency has been linked to non-musculoskeletal conditions such as cardiovascular disease, insulin resistance and cancer [2]. However, these findings were epidemiological observations that suggest associations not causality. Nevertheless, increased awareness of the potential link between VD deficiency and negative health consequences [2] has led to mounting interest in VD status and rise in VD testing [3].VD status is obtained by measuring serum levels of the prohormone 25-hydroxyvitamin D (25OHD) which is naturally synthesized in the body by ultraviolet (UV) activation. Consensus is lacking on the optimal target for 25OHD levels and the reference ranges are based on its physiological effect on bone metabolism and muscle function. The American Endocrine Society (AES) defines 25OHD levels <20ng/mL as deficient, 20-29 ng/mL as insufficient and >30ng/mL as sufficient [4]. The Institute of Medicine (IOM) defines 25OHD levels >20ng/mL as sufficient to normalize PTH and calcium levels [5]. Outcome studies determining the relationship between 25OHD concentrations and clinical benefits are conflicting and limited to musculoskeletal health [6]. A meta-analysis showed that a threshold 25OHD level of >24ng/mL and VD doses of 700-1000 units/day resulted in fall risk reduction of 23% and 19% respectively, in adults aged above 65 years [7]. VD deficiency (<20ng/mL) is a recognized worldwide problem [8]. In Australia, 88.2% of community elderly were VD deficient [8] while in France the prevalence was 34-50% [9]. VD deficiency in Southeast Asia is also prevalent despite receiving a constant intensity of sunlight. Among Malaysian post-menopausal women, 71% of Malay and 11% Chinese women were VD deficient [10], whereas a study of Singaporean adults found that 30-85% were VD deficient with highest incidence among Malay females [11]. In the hospital setting, 57% of warded American patients were deficient [12], while in England, 83-92% of patients admitted for fracture had VD deficiency [13]. Two hospital studies in Singapore showed that 57.5% adults admitted with hip fractures [14] and 44% of elderly admitted for rehabilitation were VD deficient [15].Despite the health implications associated with VD deficiency, most authorities do not advocate the universal screening of VD levels in the general population due to high cost and dearth of evidence that testing improves outcomes [16-19]. The AES identified clinical conditions in which affected individuals would benefit from VD testing [4]. The cost burden of VD testing is a concern in many developed nations. The annual cost of 25OHD testing in Australia rose from $1.0 million (AUD) to $95.6 million (AUD) in 2000 and 2010 respectively [20]. Similar trends were observed in UK, Canada and United States [17,21]. Aside from financial harm, non-indicated testing in individuals can lead to unnecessary disease labelling, overdiagnosis and low value care [22].To justify the cost of testing, individuals with VD deficiency should be promptly treated. Clinical inertia to manage VD deficiency following testing, can expose individuals to prolonged musculoskeletal pain, weakness and risk of falls and fractures.There is paucity of literature for VD ordering and replacement patterns among doctors in Asia. This study aims to consolidate current VD testing and replacement trends in the largest tertiary medical center in Singapore. As the healthcare landscape moves towards value-based care, insight in VD testing and replacement patterns can assist to develop effective strategies to ensure judicious testing and better patient outcomes.
2. Methods
- Study Design and EthicsThis was a single-center retrospective data extraction analysis performed in Singapore General Hospital. The study was approved by the institution’s review board (IRB No. 2017/2584). ObjectivesThe primary objective was to assess temporal trends in annual 25OHD tests. The secondary objectives were to assess appropriateness of tests, sub-analysis of adults with repeated test for VD status change, replacement rates, VD regimens and factors associated with sufficient levels at retest.Inclusion CriteriaAll adults (>21 years) who had a VD test between 1 January 2011 to 31 December 2016 were identified.Exclusion CriteriaAdults with invalid assays or samples collected after 20 December 2016 (due to change in VD assay test) were excluded. Adults with no documented diagnosis and did not meet our definitions for index tests and retests were excluded (Appendix 1).Data CollectionAdult demographics, co-morbidities, 25OHD test information (sampling date, results, care setting) and drug orders were electronically extracted from medical records (Appendix 2,3).Definitions and ProceduresVD status was defined according to AES [4]. We chose 25OHD threshold of >30ng/mL for sufficiency since it is the desired level for high risk population [4].Index 25OHD test was defined as the first 25OHD assay performed for individual without prior tests done in 365 days. Retest was defined as VD assays repeated within 365 days after the index test. Adults who died within 365 days following the index test were excluded to prevent bias from underrepresentation of retest frequency. Baseline adult characteristics and drug regimens were collected at the time of the index test. Clinical conditions requiring 25OHD testing by AES [4] (chronic kidney disease (CKD), chronic liver disease, malabsorption syndromes, fractures, falls, metabolic bone disease, hyperparathyroidism, obesity (BMI >30mg/m2), granuloma-forming disorders, myalgia, fatigue) were identified using International Classification of Diseases (ICD 9,10). Estimated glomerular filtration rate was calculated using the four-factor Modification of Diet in Renal Disease (MDRD) study equation. Adults on chronic therapy with VD regimens and drugs associated with VD deficiency including steroids (except for physiological replacement) [23], anticonvulsants and antiretroviral drugs [4] were identified. Chronic therapy was defined as uninterrupted prescription orders (<14 days order intervals) for >90 days duration. Regimens containing VD2 were converted to VD3 equivalent doses by dividing by three [24].Time to VD replacement was defined as days to first prescription order, within 180 days post index 25OHD test. Individuals were considered not replaced if no VD regimen was ordered, or repeated test ordered within 180 days. We considered 180 days as the threshold, because the clinical relevance of the test was redundant if VD therapy was not prescribed when levels were suboptimal. VD assaySerum 25OHD levels were measured using DiaSorin® 25OHD 125I radioimmunoassay [25] in the institution’s laboratory until 20 December 2016. Statistical AnalysesDescriptive analyses and linear regression were used for annual trends in 25OHD tests. Categorical and continuous variables were evaluated for trends using chi square test and linear regression respectively. McNemar test was used to assess the change in VD status between the index and first retest comparing between adults with low (<30ng/mL) and sufficient (>30ng/mL) levels at index test. We performed multivariate logistic regression to examine factors associated with reaching sufficient levels at retest. Variables (age, gender, ethnicity, clinical risk conditions, baseline 25OHD levels and VD doses) were reported as adjusted odds ratio (AOR) with 95% confidence interval (CI) representing the odds of achieving sufficient levels at retest. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 25.0.
3. Results
- Adult demographicsA total of 15,605 adults with 23,502 serum 25OHD tests performed from 2012 to 2016 were analyzed (Diagram 1). There was a 140% increase in number of tested adults from 1,795 to 4,244 adults in 2012 and 2016 respectively. The average age (SD) of adults was 66 (16.2) years and were predominantly female (10,384, 66.5%). Approximately 78% (12,188) were Chinese, 9% (1,352) Malays and 9% (1,366) Indians. At index test, 32.3% of adults were VD deficient, while 42.1% and 25.6% were VD insufficient and sufficient respectively (Table 1). The mean index (SD) 25OHD levels was 24.4 (9.5) ng/mL. There were no differences in the annual change for VD status and mean 25OHD levels (p=0.56, p=0.67 respectively).
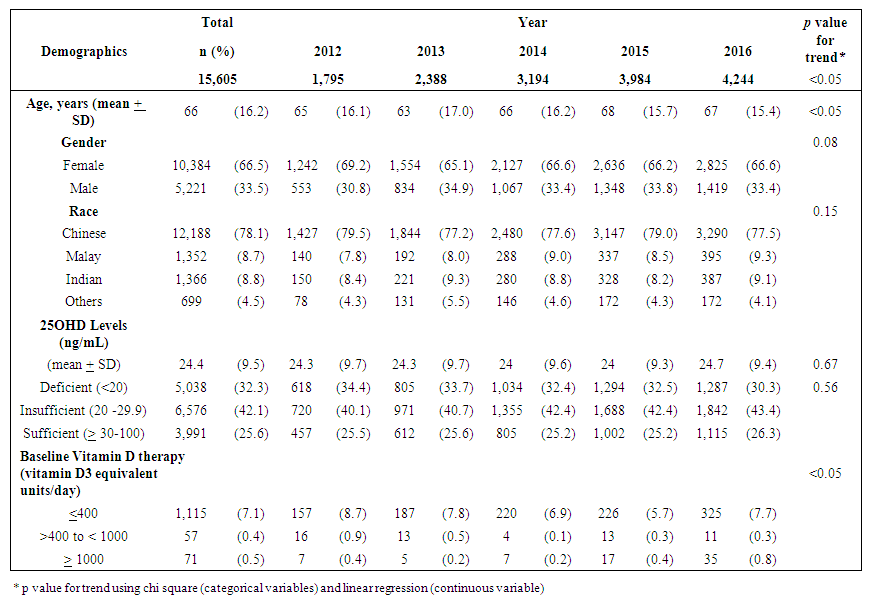 | Table 1. Patterns of demographics of adults with 25OHD tests performed over 2012 to 2016 |
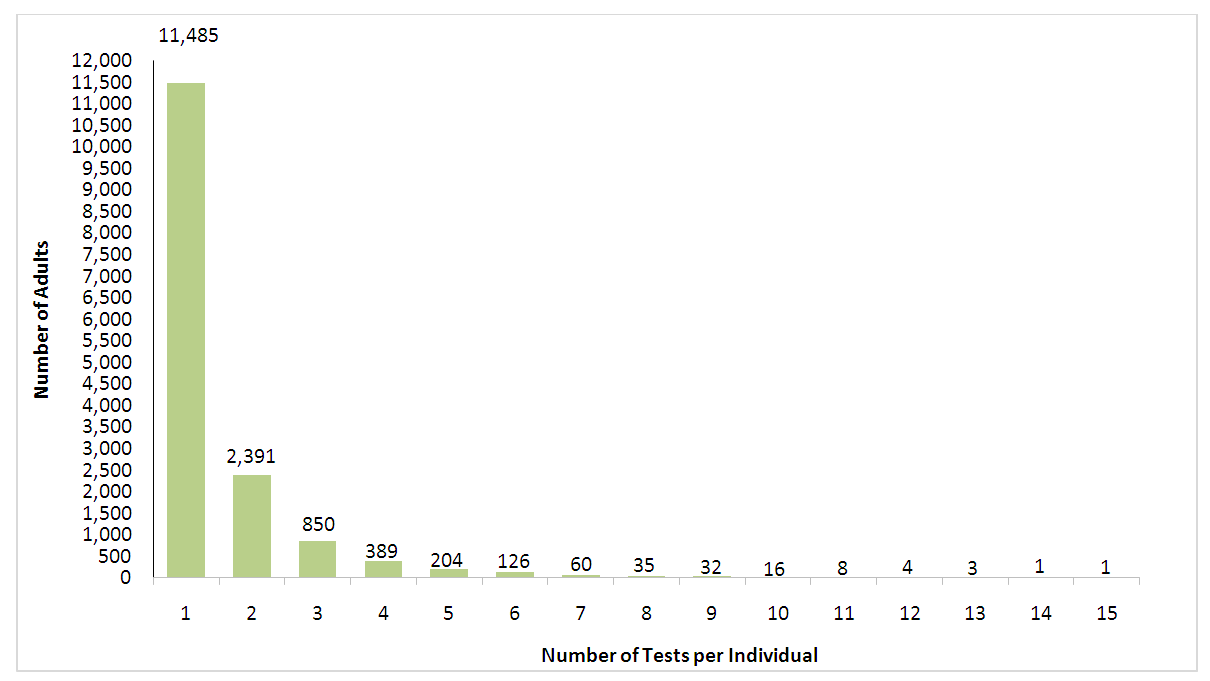 | Graph 1. Distribution of 25OHD tests among adults by number of tests per individual for 2012-2016 |
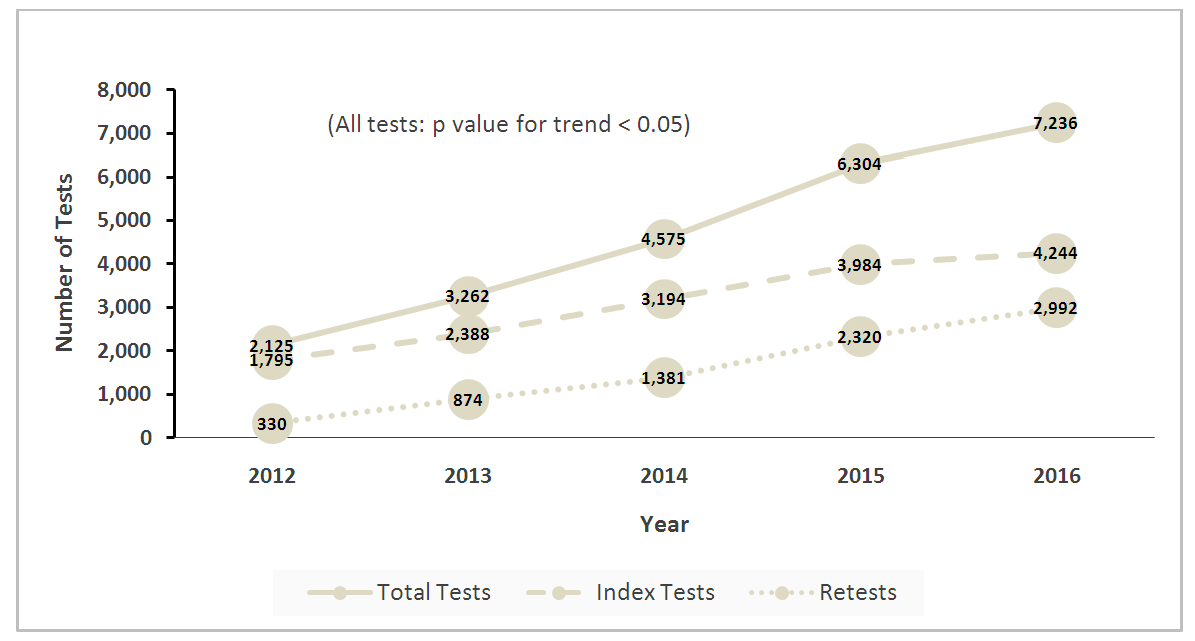 | Graph 2. Annual trends in test volumes for total tests, index tests (defined as nil test done 365 days prior) and retests (defined as test done within 365 days post index test) |
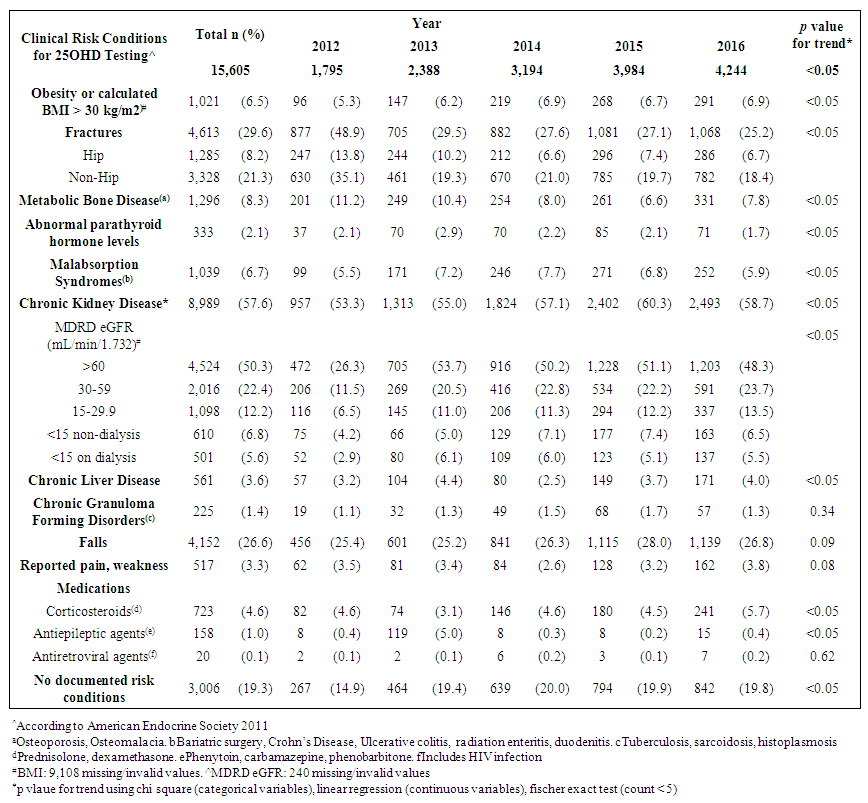 | Table 2. Trends of clinical risk conditions among adults tested for 25OHD levels from 2012 to 2016 |
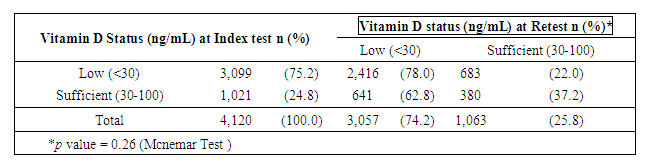 | Table 3. Vitamin D status change of Adults between 2 consecutive tests |
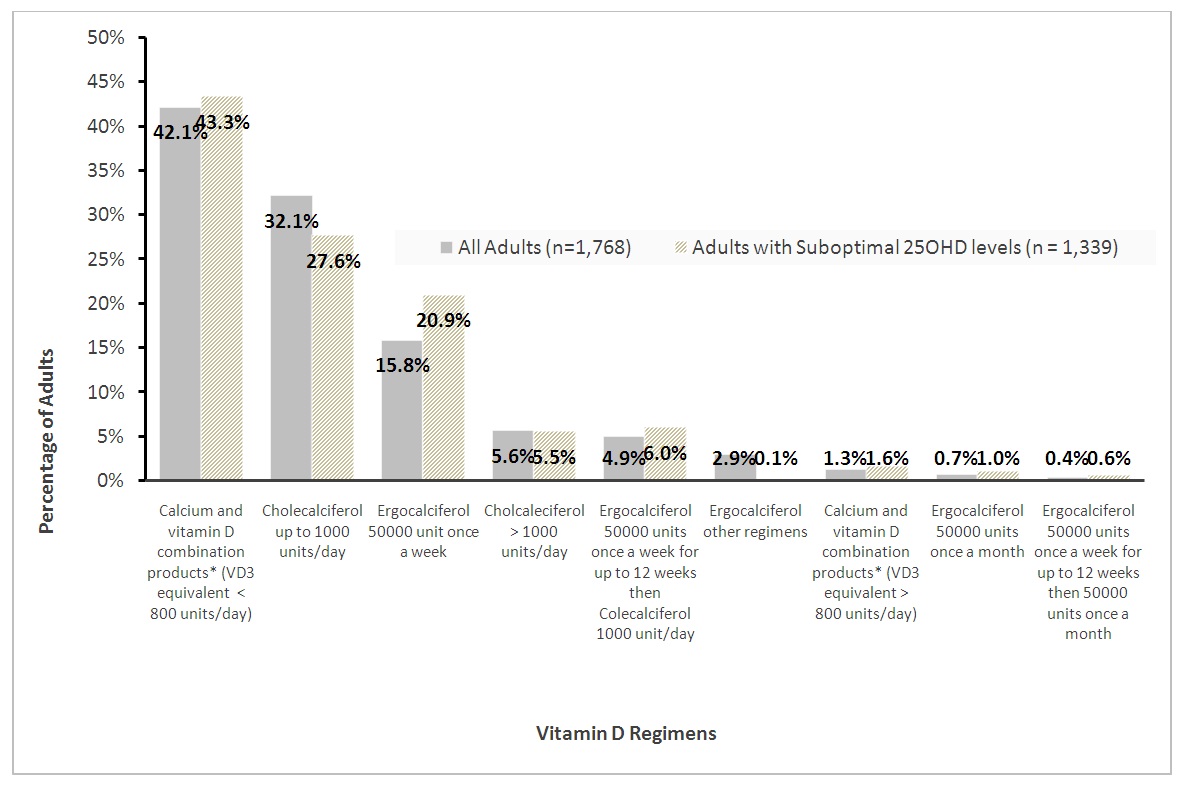 | Graph 3. Pattern of Prescribed vitamin D regimens for replacement in adults with 2 serum 25OHD tests |
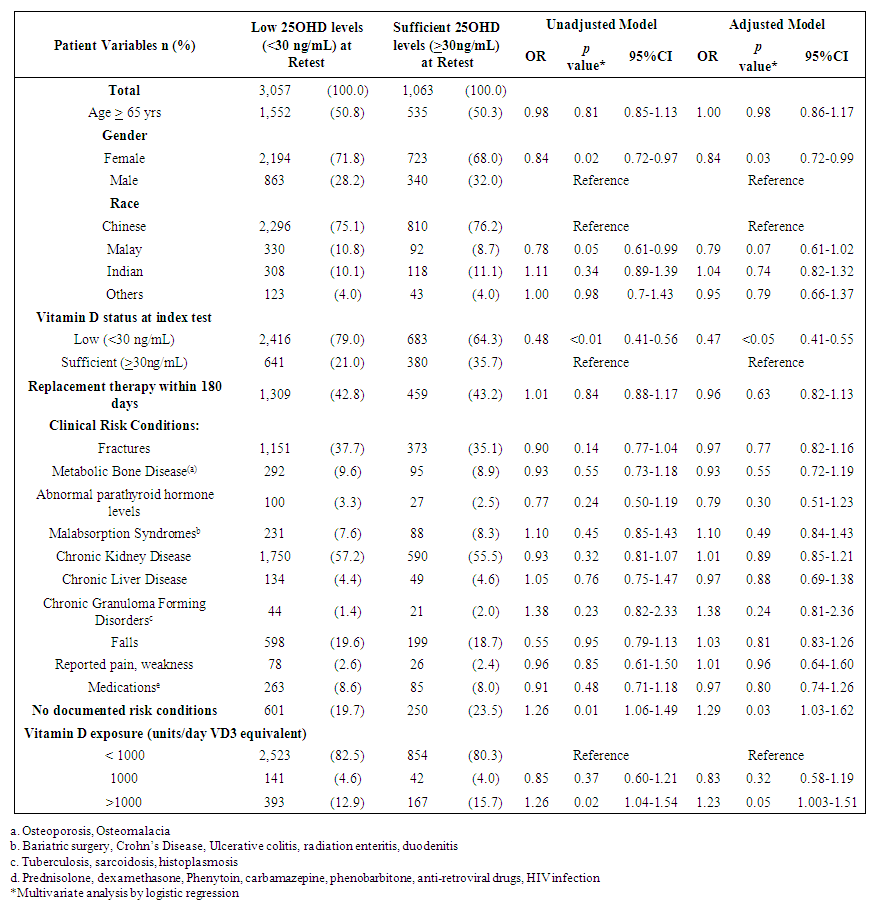 | Table 4. Factors that affect adults with sufficient levels at retest |
4. Discussion
- This study reported the patterns of 25OHD testing and VD replacement patterns. To our knowledge, this is the largest study conducted at a tertiary setting in the Asia Pacific region. The large data set permitted us to derive temporal trends and gain deeper insight into patterns and associations in our center’s current practice of VD testing and prescribing. We observed a modest rise in the number of 25OHD tests from 2012 to 2016 and testing was indicated for the majority. Among the adults with 2 tests, three quarters did not reach or maintain sufficient levels at the second test, and majority did not receive VD replacement within 180 days. Female gender, low baseline VD levels were factors less likely to achieve sufficient levels at retest, whereas, no clinical risk conditions and high VD3 doses (>1000 units/day) were more likely to attain sufficiency status. We observed that most adults tested were Chinese (78.1%), female (66%) and mean (SD) age 66 (16) years. Post-menopausal women and the elderly are at risk for osteoporosis and likely targeted for testing. The study’s ethnic distribution similarly reflected the population [26]. Our study observed 32% adults as VD deficient which was lower compared to a local multiethnic study which reported 47.8% [27]. The higher proportion of Malay and Indians in their study may account for this, since VD deficiency among these groups are more prevalent compared to the Chinese [11]. This study demonstrated an increase in annual 25OHD test volumes and number of adults being tested which was similarly reported, [16,17,28]. However, the 2.4 fold increase of 25OHD assays over 4 years was lower than other countries. Australia reported a 94 fold increase in 25OHD testing from 2000-2010 [16], while France [28] and UK [21] reported an 8 and 6 fold increase in testing over 6 and 4 years respectively. We attributed this to the tertiary nature of our hospital, where tests were ordered by specialists. In contrast, the international studies were multi-centered and included tests ordered by general practitioners, which represented a higher proportion of practicing physicians [16]. The proportion of adults with repeated 25OHD tests in our study was 26.4%. This is similar to the rate reported in a French study which was 20% [28], but lower than an Australian study where 42.9% of individuals had multiple tests [16]. Our study demonstrated a 9 fold increase in retesting over 4 years which was also similar to the French study [28]. Despite an uptrend in repeated tests, our study showed minimum VD status improvement at retest which corroborated a previous study [18]. In our study, majority of adults with low levels at baseline did not reach sufficient levels at the next test. For adults with sufficient levels at baseline, sufficiency status was not sustained at retest. These findings may suggest gaps in clinical management following 25OHD index test to optimize VD status. We observed a wide variation in time intervals between tests which is similar to another study that observed test intervals of 300-400 days18. Bailey et al. showed that remaining VD deficient may have cost implications such as increased cost in health services in both outpatient and inpatient settings [30].Majority of adults in our study had co-morbidities which impair VD bioavailability through mechanisms involving reduced production, malabsorption, increased catabolism or accelerated VD binding protein losses [2,3]. CKD is an associated risk factor for hip fractures [31] and was a prevalent condition among the adults in our study. Higher repletion doses of VD is needed in this high risk group to reach the target level [4]. AES recommends loading with 50,000units of vitamin D2 or D3 once a week for 8 weeks to achieve 25OHD levels of 30ng/mL followed by maintenance with cholecalciferol 1,500-2,000units/day [4] to sustain desired levels. International Osteoporosis Foundation (IOF) recommends repeating a 25OHD assay in 3 months in high risk individuals, to ensure that 25OHD level of 30ng/mL is achieved [32].Our study detected only 32.5% of adults with suboptimal 25OHD levels receiving VD replacement therapy within 180 days. Other studies also revealed a lapse in VD replacement following suboptimal serum 25OHD levels with 31-60% patients not repleted [34-36]. To justify for the cost of VD assay orders, prompt VD replacement should be taken to improve VD status.We elucidated possible barriers to VD replacement. Disparity in serum 25OHD thresholds to initiate replacement among prescribers, especially if 25OHD levels fall between 20-29ng/ml which is sufficient by IOM guidelines but insufficient according to AES. Addition of a complex regimen involving weekly loading followed by daily maintenance, can compound pill burden issues for patients struggling with polypharmacy. Patients may prefer non-pharmacological approaches such as increasing dietary intake of VD rich foods and longer sunlight exposure. Patients may be lost to follow up post 25OHD assay when care continuity is disrupted during transition. Long turnaround times for 25OHD result values (5-7 days) delay doctors to review results before patients are discharged from hospital.Our study observed that current VD prescribing practices were not adequate to raise serum 25OHD to sufficient levels. We identified an array of VD regimens prescribed among adults with repeated tests, with the most common regimen being combination products with calcium at VD3 doses <800 units/day. Despite a heterogenous study population, the majority had risk conditions, requiring higher repletion doses of at least 1000units/day [4]. Only a third of the subgroup of adults with suboptimal levels were prescribed cholecalciferol 1000units/day and 20% were ordered ergocalciferol 50,000units/week which are regimens recommended for VD repletion [4,5]. One study also observed daily cholecalciferol being more frequently prescribed than weekly ergocalciferol likely being a simpler regimen and easier for adherence [35].In our study, female gender and low baseline 25OHD levels were inversely associated with achieving sufficient 25OHD levels at retest. Other studies also reported that women were less likely to achieve sufficiency status than men [11,27]. Women have lower VD bioavailability than men due to their higher body fat composition, causing the VD sequestering in adipose tissue and preventing bioactivation [37]. Women in Asia tend to avoid sunlight exposure and cloth themselves extensively for cultural reasons, hence limiting cutaneous VD activation. Our study found a positive association between high VD doses (VD3 >1000units/day) and achieving sufficient levels at retest. Other studies have supported linear but varied dose-response relationships between 25OHD levels and VD doses [24,36,38]. One study observed that daily doses of cholecalciferol 600-1,100units raised 25OHD levels by 8-9.1ng/mL. Another study observed that every 1000units cholecalciferol/day increased the 25OHD level by 10ng/mL (range 7-11ng/mL) [38]. The varied dose-level response may be multifactorial. Factors such as sun exposure, percentage body fat, diet and presence of risk conditions may affect 25OHD levels. Our study had several limitations. Being a single-centered study, VD testing trends cannot be generalized to other medical centers in Singapore. We only captured 25OHD tests ordered by hospital specialists and not general practitioners whose 25OHD tests ordering patterns likely differ [16]. We only analyzed data captured electronically and medication records may be incomplete due to omission of supplement use [39]. The extent of VD replacement may be underreported as doctors can verbally instruct patients to buy their own VD supplements. Other discrepancies not captured are patient medication taking habits and adherence. Another limitation is we didn’t capture lifestyle factors that can influence VD status such as dietary VD intake and sun exposure [19,40]. To develop a value-based strategy for VD testing, high risk individuals should be targeted for testing and replete to achieve 25OHD level of 30ng/mL to optimize musculoskeletal health and prevent falls [41]. As individual dose responses vary, we suggest repeating a 25OHD assay in 3 months to ensure desired levels are reached, and maintenance doses (VD3 >1000units/day) to sustain levels. Empiric VD supplementation can be considered over VD testing in low risk individuals due to its low treatment cost and toxicity risk.
5. Conclusions
- While our study observed an uptrend in VD test volumes and most being medically indicated, majority of adults remained at suboptimal levels despite repeated testing. Associative factors identified which were inadequate repletion VD doses, low baseline VD level and female gender are potential focus for improvement strategies to efficiently utilize VD assays, improve patient VD levels and outcomes.
Appendix
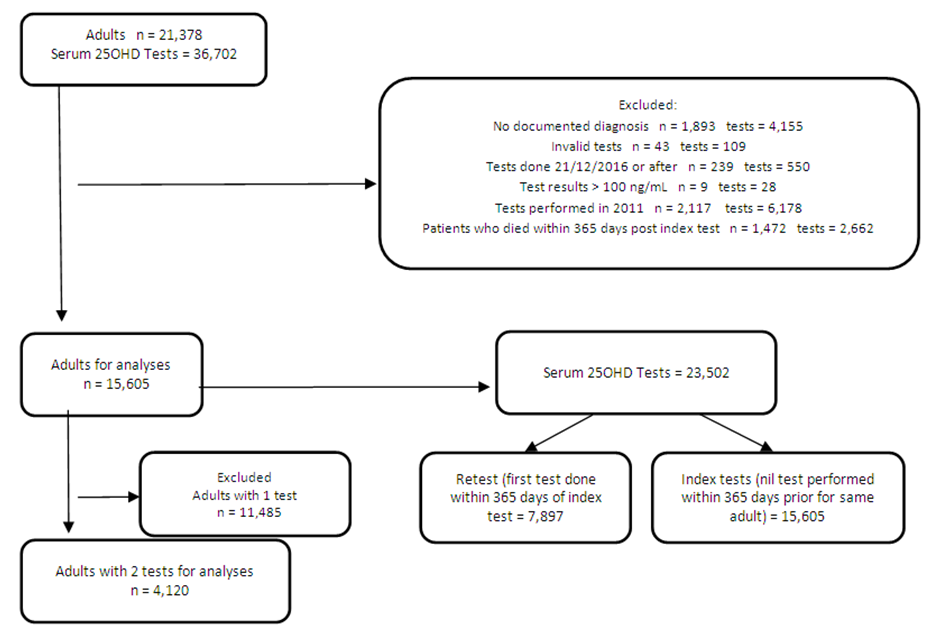 | Appendix 1. Adult and test criteria and selection |
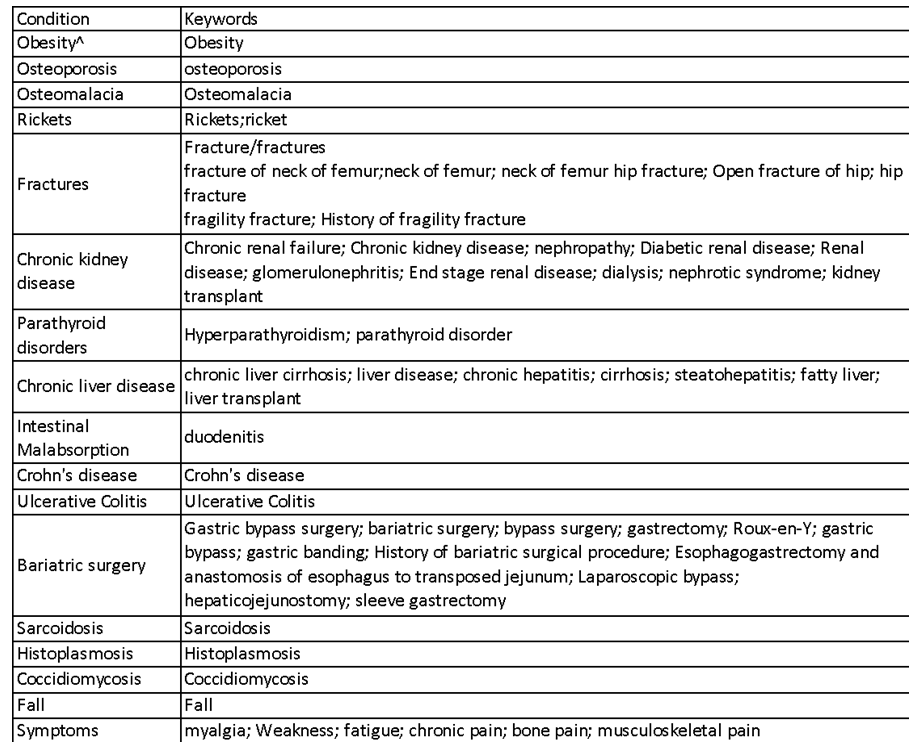 | Appendix 2. Table of clinical conditions keywords |
 | Appendix 3. Drugs associated with vitamin D deficiency extracted from prescription orders |
 | Appendix 4. Vitamin D containing products for replacement therapy extracted from prescription orders |
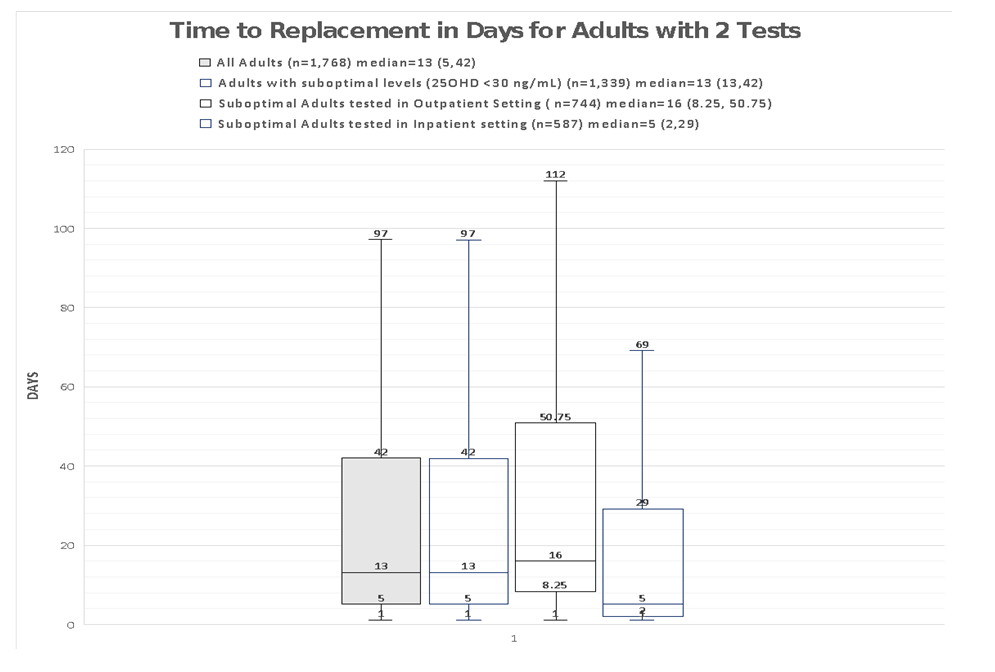 | Appendix 5. Time to replacement for Adults with suboptimal serum 25OHD levels (<30 ng/mL) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML