-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2020; 9(2): 23-29
doi:10.5923/j.ijim.20200902.01
Received: July 2, 2020; Accepted: August 3, 2020; Published: August 15, 2020

Role of Non-Invasive Ventilation in Management of H1N1 Influenza Cases: A Study from Western India
Minal Shastri 1, Amrin Kharawala 2, Smeet Bhavsar 1, Jay Jani 2, Dhwani Thakar 1, Kalpita Shringarpure 3
1Department of Medicine, SSG Hospital and Medical College Baroda, Vadodara, India
2SSG Hospital and Medical College Baroda, Vadodara, India
3Department of Preventive and Social Medicine, SSG Hospital and Medical College Baroda, Vadodara, India
Correspondence to: Amrin Kharawala , SSG Hospital and Medical College Baroda, Vadodara, India.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: H1N1 Influenza is a contagious disease, often amounting to epidemics globally. Severe cases require ventilatory support as a life saving measure. The role of Non-Invasive Ventilation (NIV) as an alternative to invasive ventilation in these patients is increasing. In this study, we aim to study the clinical profile and outcome of patients on NIV support at a tertiary care center in India. Methods and design: In this retrospective study, 108 RTPCR confirmed H1N1 positive patients required NIV at a tertiary care center over a period of ten years (2009-2019). Data was collected according to a standardized proforma which included parameters like demographics, clinical features, examination findings, comorbidities, X-ray and laboratory reports. Subsequently patients were classified into two groups – those who recovered purely on non-invasive mode of ventilation and those who were shifted to the invasive mode. Chi-square test and Mann Whitney U test were used for qualitative and quantitative data respectively. A p-value of less than 0.05 was considered significant. Results: From a total of 528 hospitalized patients with H1N1 Influenza, 108 patients were subjected to non-invasive ventilation (NIV) and 22 (20.04%) of them were subsequently shifted to invasive ventilation. Co-morbidities were present in 52.8% (57/108) of the cases, most common being hypertension, followed by pregnancy. A higher pulse rate, lower respiratory rate, lower oxygen saturation along with a high median SGOT, ALP and serum bicarbonate levels were significantly associated with the patients being shifted from NIV to invasive ventilation. Conclusion: NIV was effective in preventing invasive ventilation in 86 (79.06%) of the 108 patients with H1N1 infection, while 22 (20.04%) patients still required intubation despite NIV. Hence, NIV can be considered to be used as an alternative to invasive ventilation in eligible patients with H1N1 influenza.
Keywords: Non-invasive Ventilation, NIV, BiPAP, H1N1 influenza
Cite this paper: Minal Shastri , Amrin Kharawala , Smeet Bhavsar , Jay Jani , Dhwani Thakar , Kalpita Shringarpure , Role of Non-Invasive Ventilation in Management of H1N1 Influenza Cases: A Study from Western India, International Journal of Internal Medicine, Vol. 9 No. 2, 2020, pp. 23-29. doi: 10.5923/j.ijim.20200902.01.
Article Outline
1. Introduction
- Influenza, an acute viral infection of the respiratory tract, is caused by a single stranded, segmented RNA virus belonging to the orthomyxo family. In 2009, a major shift in the hemagglutinin and neuraminidase segments gave rise to the H1N1 strain. After leading to a pandemic in 2009 globally, the number of H1N1 cases are gradually decreasing. In India, 27,236 cases were confirmed in 2009, which fell to 20,604 in 2010. [1] A reemergence of H1N1 influenza cases was noted in 2015, which saw a total of 31,974 confirmed cases with 1895 deaths. [1] Once again, in 2018 there were 15,266 cases and 1113 deaths. As on 5th May 2019, 24,456 cases of H1N1 have been reported with a predominance in the states of Gujarat and Rajasthan, in India. [2]Most people who get sick with the flu have mild illness and recover without medical care and antiviral drugs in less than two weeks. Some patients, however, are more likely to get severe disease with complications that can result in hospitalization and sometimes death. These include adults above 65 years, young children, pregnant women, people with HIV/AIDS, diabetes, heart diseases, stroke, cancer, obesity, asthma and children with neurological conditions. [3] Based on the CDC categories, patients of Category C are admitted. [4] Non-invasive mechanical ventilation(NIV) is widely used in the acute care setting for acute respiratory failure (ARF), which is one of the most common causes of death (mortality rate 40-46%) [5]. Although The European Respiratory Society/American Thoracic Society recommends clinical application of NIV in Exacerbation of COPD, Cardiogenic Pulmonary edema, De novo hypoxemic respiratory failure, immunocompromised patients, Chest Trauma, Palliation, Post-operative care, weaning and post extubation, they have concluded that, as a general rule, NIV should not be recommended as an alternative to invasive ventilation in patients affected by H1N1. [6] Different studies have reported a range of 62-100% of patients requiring mechanical ventilation (MV), with a mortality rate varying from 16 to 58%. [7-15] Although frequently used, invasive MV has several complications, ranging from those associated with the placement of an endotracheal tube to ventilator-associated pneumonia, baro-trauma and oxygen toxicity. Positive-pressure ventilation can decrease preload, stroke volume, and cardiac output and also affect renal blood flow and function, resulting in gradual fluid retention. The incidence of stress ulcers and sedation-related ileus is also increased when patients receive invasive mechanical ventilation. [13]There are very limited number of studies which have documented the outcome of H1N1 influenza patients who were subjected to NIV. Even today, most hospitals prefer to directly start invasive ventilation without even giving a trial of NIV due to the scarcity of proven benefit and enough evidence. This study is one of its kind, having a large cohort of 108 adult patients, over a course of ten years, from a tertiary care hospital, that analyses the outcome of H1N1 patients subjected to NIV during the course of hospitalization.
2. Materials and Methods
- This is a retrospective record-review based study, conducted at Sir SayajiRao General Hospital, a tertiary care center in Vadodara, India. The inclusion criteria include: RTPCR confirmed H1N1 patients, Category C [17] patients older than 18 years of age and those patients who were subjected to NIV. The exclusion criteria included: all patients with symptoms of influenza who did not test positive for the H1N1 strain via rt-PCR, patients with H1N1 influenza falling in Category A and B, patients who needed nasal oxygen, NRBM, patients who had absolute contraindications for NIV like Cardiorespiratory arrest, extreme psychomotor agitation, severe hemodynamic instability, non-hypercapnic coma and multiple organ failure [14], those who were already intubated and those who directly needed invasive mode of ventilation.To confirm the cases of H1N1 influenza virus, a real-time RT-PCR testing was performed on nasopharyngeal swab samples of all patients with suspected signs of influenza A, in accordance with the published guidelines from the Center for Disease Control (CDC). Data was collected on a cohort of 528 hospitalized patients, who tested positive for H1N1 Influenza via rt-PCR from June 2009 to May 2019. H1N1 seropositive patients can be classified under following categories: Category A –Mild fever, cough, sore throat with or without headache, body ache, diarrhea, vomiting. Category B1 – Symptoms of category A plus high-grade fever plus severe sore throat.Category B2 –symptoms of category A plus High-Risk Group women, patients >65 yrs., patients with underlying lung disease heart disease, kidney disease, cancer, HIV/AIDS.Category C –Symptoms of category A and B plus following Breathlessness, Chest pain, Drowsiness, Low BP, Sputum with blood, bluish discoloration of nail, irritable child, worsening of underlying medical disease. [4]Only Category C patients are tested and admitted for H1N1 Influenza. These are the cases that are reported at the national level and hence this study focuses only on patients who fall under category C. Amongst them, 108 patients satisfied our inclusion criteria. Majority of patients presented with symptoms like – fever, cough, sore throat, body aches, chest pain and dyspnea. They were treated with tablet Oseltamivir as per guidelines of the Government of India (150 mg twice daily for at least 5 days) [16] Certain treatment decisions like the need, duration and settings of NIV, the decision to wane from NIV or to intubate and shift to invasive ventilation, along with the increase in the number of doses of Oseltamivir and the addition of corticosteroids, were made on the basis of the clinician’s acumen. The data was collected after reviewing the files of all H1N1 positive patients collected from the Medical Records and Statistical Department of SSG Hospital. The following parameters were recorded on admission: name, age, sex, demographic data, comorbidities, time from illness onset to hospital admission, clinical symptoms and vitals. A thorough clinical examination was performed with a detailed focus on the respiratory system. Laboratory investigations conducted were: Complete Blood count, biochemistry profile, Arterial Blood Gases (ABG) analysis, and chest X-ray. The number of days that these patients were subjected to NIV and the need for invasive ventilation was also recorded. The end-point of the study was survival or death.Acute respiratory failure was suspected in patients who had tachypnea with RR >30, hypoxia with spo2 <80 and use of accessory respiratory muscles. Out of these the conscious, alert and hemodynamically stable patients were subjected to non-invasive ventilation in form of BiPap therapy. Altered and hemodynamically unstable patients were intubated promptly and subjected to invasive ventilation. BiPap therapy was considered successful if there was improvement in oxygen saturation, reduction in respiratory rate (<30), and no use of accessory respiratory muscles within 3 hours of initiation. If the patients failed to improve on BiPap, they were promptly intubated and shifted to invasive mechanical ventilation. Due consent was taken before initiation of non-invasive and invasive therapy.Patients were explained proper usage of BiPap mask to prevent any leak.Drager Savina RS232 ventilator was used to provide non-invasive ventilation in BiPap mode with a standard sized full face mask.In this study, the cohort of 108 patients were divided into two major groups – those who survived on NIV(86 patients) and those who had to be transferred to invasive ventilation (22 patients). This was done in order to study the difference in clinical and laboratory parameters and their effect on the outcome of all patients subjected to NIV.This study, analyses the clinical and biochemical profile in patients who were successfully treated only on non-invasive ventilation and of those who still required invasive ventilation to improve. Through this, we aim to find out the rate of success of NIV in patients of H1N1 influenza and the patient parameters that might affect it.Association between these factors and need for shifting to invasive ventilation was studied using Chi-square test for categorical variables and Mann Whitney U Test for quantitative variables. A p value of 0.05 was considered statistically significant.
3. Results
- During June 2009 to May 2019, 108 confirmed H1N1 cases with acute respiratory failure were subjected to non-invasive BiPaP ventilation at the SSG Hospital, a tertiary care hospital, affiliated to Medical College in Western India. From a cohort of 108 patients who were subjected to NIV, 20.4% (N=22) of the patients had to be shifted to mechanical ventilation due to deterioration of condition despite providing BiPaP ventilation. The overall mortality rate was 20.4%.
3.1. Patient Characteristics
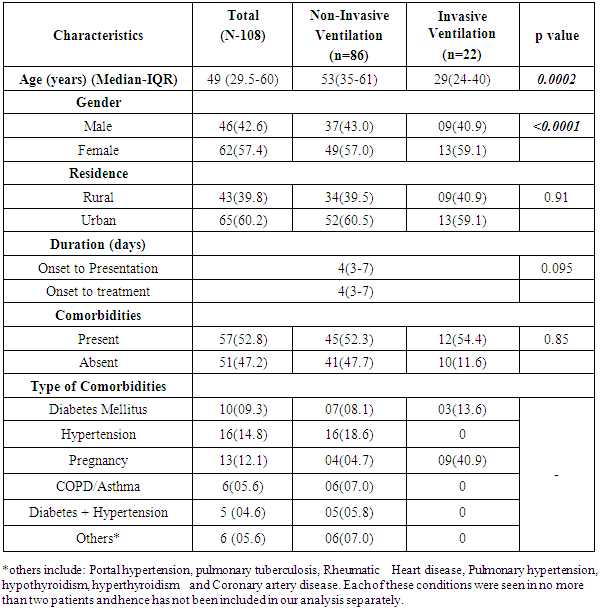
- There were 62 females (57.4%) and the Median age (Interquartile Range-IQR) was 49 (29.5-60) years. 62% of the cases were from urban areas (Table 1).
|
3.2. Clinical Characteristics
- As per treatment protocol, the H1N1 positive patients with acute respiratory failure were initially put on Non-Invasive ventilation (NIV), after which 20.4% (22/108) of the patients were shifted to Invasive ventilation, based on their clinical situation. The median (IQR) duration from onset to presentation and treatment for H1N1 was four (3-7) days. Co-morbidities were present in 52.8% (57/108) of the cases, most common being hypertension followed by pregnancy (16 and 13 of the cases respectively) (Table 1).
3.3. Clinical Profile
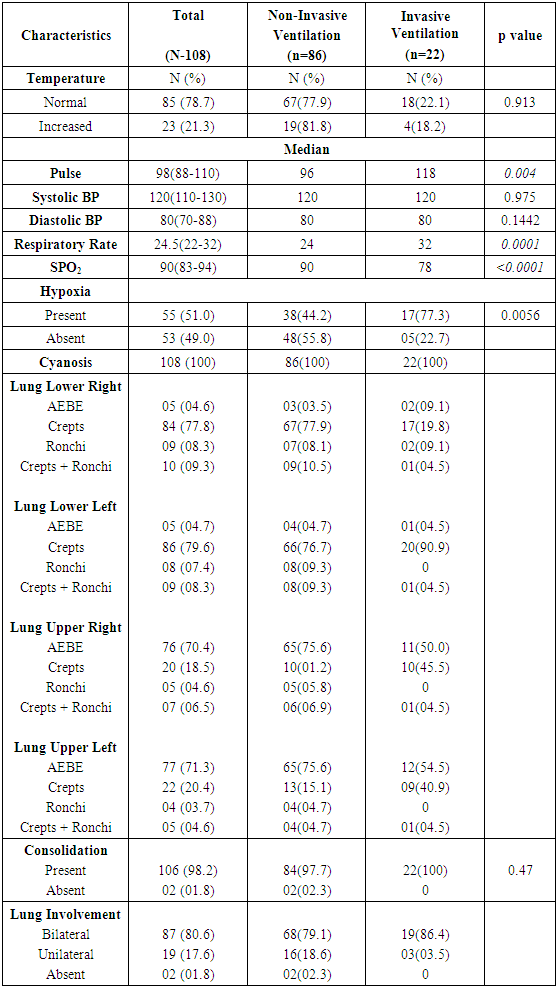
- The clinical characteristics of confirmed H1N1 cases have been shown in Table 2. Bilateral lung involvement was seen in 87/108 (80.5%) patients and consolidation was present in almost all the patients, 98.2% (106/108). Cough (101/108), fever (95/108) and dyspnea (84/108) were the most common symptoms experienced by the patients (Figure 1).
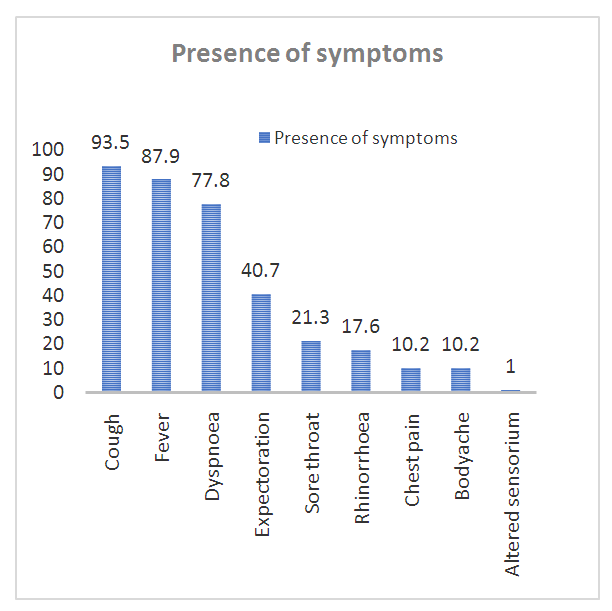 | Figure 1. Symptoms experienced by confirmed H1N1 cases admitted in Tertiary care hospital during June 2009 to May 2019. (Original) |
3.3.1. Factors Associated with Invasive Ventilation
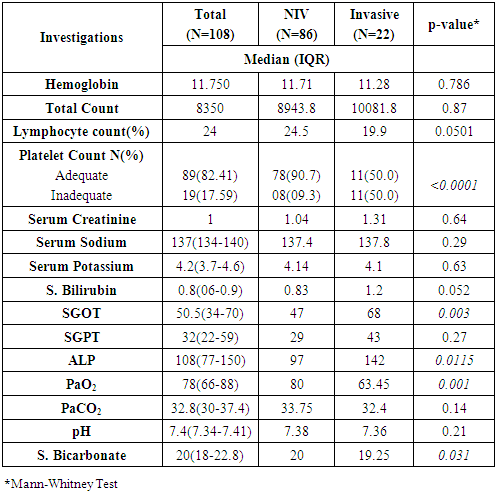
- A higher median age of patients, male gender and early onset of presentation was significantly associated with non-invasive ventilation (p=0.0002, p<0.0001, p=0.095 respectively) (Table 1). None of the symptoms was statistically different in the patients who had to be put on invasive ventilation. A higher pulse rate, lower respiratory rate and lower oxygen saturation was significantly associated with the patients being shifted from NIV to invasive ventilation (p=0.004, p=0.0001, p<0.0001 respectively) (Table 2).
|
|
3.4. Interventions and Outcomes
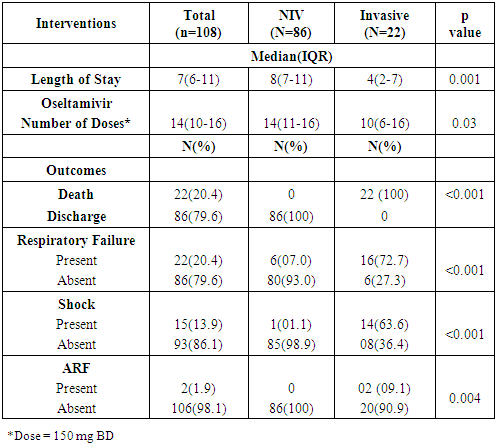
- The median (IQR) length of stay of confirmed H1N1 cases was 7(6-11) days (Median stay was higher in those patients on NIV 8 (7-11) days, p=0.001). The median doses of oseltamivir were higher among NIV intervention patients 14 (11-16) as compared to invasive interventions 10 (6-16) (p=0.03) (Table 4). The median (IQR) days of invasive ventilation were 5(3-7) days, with the outcome being death for all the 22 patients being put on invasive ventilation (p<0.001). Respiratory failure, shock and acute renal failure were significantly higher in the patients put on invasive ventilation (p<0.001, p<0.001, p=0.004 respectively) (Table 4).
|
4. Discussion
- This study has been carried out on all patients who tested positive for H1N1 Influenza via RT-PCR, over a period of ten years in one of the largest tertiary care hospitals in Western India. Through a detailed, retrospective analysis of 108 Category C patients who were subjected to NIV during the course of their hospitalization, we have tried to find out whether a trial of NIV should be given to patients instead of directly subjecting them to mechanical ventilation. This study is unique in a way that it is the first study that tries to assess the credibility of NIV in H1N1 patients by reviewing data ranging back over a period of ten years.While India has not seen many studies to prove the efficacy of NIV in H1N1 patients, there are still a few studies conducted in other parts of the world that share a similar conclusion as ours. [18-21]In our study, 20.04% (22/108) patients had to be shifted to invasive ventilation, while 79.06% (86/108) patients recovered on NIV. This is in line with quite a few other studies carried out in recent times. In fact, our study has shown a considerably large cohort of patients recovering only on NIV, hence preventing the unnecessary use of Invasive ventilation in all patients with H1N1 Influenza. A study published in American College of Chest Physicians as recent as October 2018, found that of 51 patients with respiratory failure who were eligible for NIV, 26 patients (50.9%) recovered on only NIV, while 23 patients had to be intubated. [18] A similar study conducted at the advent of the H1N1 pandemic, in China, also concluded that introducing BiPaP in patients with pneumonia due to H1N1 influenza suffering from acute respiratory failure can decrease the rate of invasive mechanical ventilation. [19] While there are not many articles with a large cohort of patients on NIV to prove its efficacy in H1N1 patients, there have been multiple case reports done in Bahrain [20] and Brazil [21] which warrant the same. On the other hand of the spectrum, there are a few studies which still do not advent the use of NIV in place of invasive mechanical ventilation in patients with H1N1 Influenza. [8] A study conducted by Rello and his colleagues documented the first 32 patients who suffered from H1N1 Influenza (PIAH1N1) in Spain in 2009. [8] Out of the eight (33.33%) patients who received NIV in their ICU, six (75%) had to be shifted to invasive ventilation and two (33%) died. [8] Through this they have proposed that despite the fact that NIV temporarily improves oxygenation and reduces the work of breathing, it may not change the actual course of the disease and hence they do not recommend it for patients with H1N1 virus infection which is either complicated by pneumonia, acute lung injury or ARDS. Despite having a similar demographic profile as our study, our final results are still different. Three studies, including the one quoted above have suggested that NIV has not been successful in critically ill patients with hypoxemic respiratory failure attributable to H1N1 virus infection. In these studies, a total of 76 patients received NIV, but 64 (84.2%) of these patients required subsequent intubation and invasive ventilation. [22] This could be due to the fact that our cohort is derived over a period of ten years, which has seen the advancement of treatment modalities in this field as well as the decrease in virulence of the virus and increase in overall immunity in the population.Through this study, we have taken one step further to try and analyze the reason for NIV failure in our cohort of H1N1 patients. On comparing the clinical features of patients who died in our setting, with an epidemiological report published by the WHO on the fatal cases that happened in Mexico in 2009, it is found that while 93% suffered from fever, 87% from cough, and 80% from dyspnea, the findings are quite similar to our setting over a period of 10 years, where in 87.96% suffered from fever, 93.52% from cough, and 77.8% from dyspnea [23]. However, the incidence of these clinical features is similar even in the group of patients who survived. Hence concluding that initial presenting complaint is not a significant factor contributing to the death in H1N1 patients. On the other hand, we have found that there is a significant increase in risk of deaths in patients with prior comorbidities, despite timely shift to invasive ventilation and introduction of corticosteroids.Since there have been almost no studies that have tried to compare the clinic-radiological profile and laboratory parameters to assess the reason for failure of NIV in patients presenting with similar clinical features, we have also delved in details of this aspect. The parameters described in the results section could be used as a basis to identify the most suitable type of ventilation and triage patients accordingly.Research has begun to progress in this direction and it is found that, with similar ventilator settings, non-invasive ventilation is as effective as conventional ventilation in improving gas exchange in patients with acute hypoxemic respiratory failure. Furthermore, the rate of serious complications, in particular those related to intubation was significantly lower in patients receiving non-invasive ventilation. [24] It is also said that successful non-invasive ventilation is also associated with shorter stays in the intensive care unit. [24] However, we have noticed a significantly longer duration of stay amongst patients who recovered on NIV. This could be due to early deaths in patients transferred to Invasive ventilation and may not specifically be due to a slower duration of treatment in patients on NIV. Having said this, it is also prudent to address the issues of aerosol contamination and transmission of the virus to healthcare workers in order to make NIV a success rather than a threat.Our study has put forth a well-documented evidence on the success of NIV. Despite this, we still need more evidence on a large scale so that NIV is not side lined as the first treatment modality in H1N1 influenza patients. Despite our large cohort and documentation of the illness over a course of ten years, a prospective approach may further add on to the body of evidence to prove the efficacy of NIV.
5. Conclusions
- Patients suffering from H1N1 Influenza, requiring ventilatory support are directly started on invasive mechanical ventilation without giving a trial of NIV due scarcity of proven benefit. However, through this study, we have proven that NIV was effective in preventing invasive ventilation in 86 (79.06%) of the 108 patients with H1N1 infection, while 22 (20.04%) patients still required intubation despite NIV. This shows that it is justified to use NIV as an alternative to invasive ventilation, or even as a first line therapy in eligible patients with H1N1 influenza who require ventilator support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML