-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2019; 8(3): 42-54
doi:10.5923/j.ijim.20190803.02

Serum Vitamin D and Parathyroid Hormone as Early Diagnostic Markers of Target Organ Damage among Hypertensive Patients
Eman F. Mohamed1, Eman M. Abdelsalam1, Shaimaa Ahmed Habib2, Walaa Mohamed Shipl3, Asmaa S. Hassan4
1Internal Medicine Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Cardiology Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
3Biochemistry Department, Faculty of Medicine (for Girls), Al-Azhar University Cairo, Egypt
4Clinical Pathology Department, Faculty of Medicine (for Girls), Al-Azhar University Cairo, Egypt
Correspondence to: Eman F. Mohamed, Internal Medicine Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

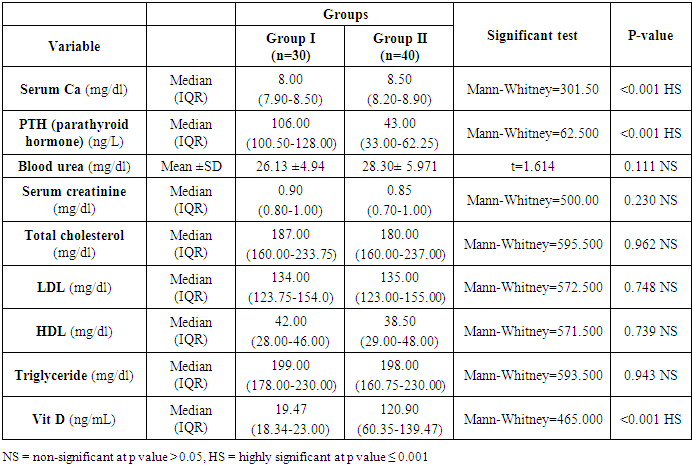
Background and Purpose: Vitamin D is fat-soluble steroids enhance intestinal uptake of calcium, iron, magnesium, and phosphate and is important for biological actions of cells in different tissues. Additionally, calcium homeostasis is associated with an increased risk of cardiovascular complications due to its vital role to play an important role in the regulation of blood pressure. The present study aims to assess subclinical LV mass and function in patients with essential hypertension and its association with vitamin D deficiency and parathormone excess. Methods: A retrospective study was carried out on 70 patients with elevated hypertension recruited from the Al-Zahraa Hospital, Faculty of Medicine (for Girls), Al-Azhar University with mean age (50.28±7.8) years. Vitamin D was measured using enzyme-linked immunosorbent assay (ELISA) technique, and parathormone was measured by chemiluminescence technique (Immulite 1000). Results: According to the level of vitamin D, the patients were divided into two groups. Thirty hypertensive patients with vitamin D deficiency as patients group (G1) and another 40 age, sex and BMI-matched hypertensive patients with normal vitamin D level as the control group (G2). Serum Ca, PTH, vitamin D has a highly significant difference among the two groups (p ≤ 0.001) without statistically significant difference for urea, serum, cholesterol, LDL, HDL, triglycerides. Conclusion: Subclinical target organ damage is significantly higher in hypertensive patients with vitamin D deficiency than hypertensive patients with normal vitamin D levels. Patients with vitamin D deficiency or excess PTH have higher LVMI, higher AMBP readings, a higher percent of non-dippers, and more impaired LV systolic function.
Keywords: Serum vitamin D, Parathyroid hormone, Diagnostic markers, Hypertensive patients
Cite this paper: Eman F. Mohamed, Eman M. Abdelsalam, Shaimaa Ahmed Habib, Walaa Mohamed Shipl, Asmaa S. Hassan, Serum Vitamin D and Parathyroid Hormone as Early Diagnostic Markers of Target Organ Damage among Hypertensive Patients, International Journal of Internal Medicine, Vol. 8 No. 3, 2019, pp. 42-54. doi: 10.5923/j.ijim.20190803.02.
Article Outline
1. Introduction
- Cardiovascular disease (CVD) mortality rates still represent 50% of non-communicable disease deaths worldwide [1] with a rapidly increased incidence among lower- and middle-income countries [2]. Moreover, hypertension is considered one of the increased risk factors for CVD, and its regulation is correlated to the prevention of the CVD and public health measures. Furthermore, a deficiency in vitamin D was also considered to be a major cause of hypertension and CVD [3-6].Vitamin D is a skin-synthesized steroid prohormone after exposure to ultraviolet radiations. It is also obtained by additional or dietary intake. Although strong evidence exists for its role in the preservation of the bone and muscles [7] the role of vitamin D deficiency in CVD conditions [8-9] has been discussed recently based on conflicting epidemiological evidence. Suitable status of vitamin D is important for the best function for many organs and tissues throughout the body, including cardiovascular (CV) system [10]. The deficiency of vitamin D is increasing globally mainly as a consequence of the environmental and lifestyle factors that limit sunlight exposure of the skin [11].Vitamin D is regarded primarily as a group of fat-soluble steroids and is accountable for the avoidance of rickets or osteomalacia by enhancing the absorption of calcium, iron, magnesium, phosphate, and zinc in the intestines [1]. However, our knowledge of the pleiotropic impacts of vitamin D has been significantly improved in various organ systems in addition to skeletal homeostasis in latest years [12]. Hypertension is a very common chronic disease and considered as a silent killer because it rarely causes symptoms. Interestingly, many animal models and observational studies have shown that vitamin D shortfall is in close relation to cardiovascular disease, particularly in the case of high blood pressure [13-18]. In addition, several intervention studies examined the effect on high blood pressure of vitamin D supplementation in patients even if their findings were uniform [19].Furthermore, the predominance deficiency of vitamin D secretion has been recorded among individuals with high hypertensive (HTN) rates in comparison to normal individuals [20], and observatory data show that low plasma vitamin D is linked to an increase in the risk of HTN [7] resulting from different risk factors such as harmful lifestyle and hereditary history, which can improve hypertrophic structural restructuring in HTN heart disease [21].The vitamin D intakes from photosynthesis or food are metabolized by 1 α-hydroxylase to 1, 25-dihydroxyvitamin D (1,25-(OH)2D, calcitriol), the organically active form in the liver to 25-hydroxyvitamin D and eventually hydroxylated in kidney proximal tubules. Intestinal calcium absorption is supported by 1.25-(OH)2D). When the intake of calcium and phosphorus is reduced by vitamin D, the levels of the hormone parathyroid (PTH) are increased [22-24]. Enough vitamin D stimulates 30 to 40 percent and 80 percent respectively in order to absorb calcium and phosphorus. No more than 10-15% of the calcium in your diet is supplied with vitamin D and about 60% of the phosphorus [25].1,25-(OH)2D reduced endothelium-dependent contractions of the aorta by decreasing cytosolic free calcium concentrations in endothelial cells [26]. 1,25-(OH)2D exerts its vasculoprotective effects by decreasing endothelial adhesion molecules, by increasing the activity of endothelial nitric oxide synthase, and through its anti-inflammatory properties. In line with these findings, vitamin D deficiency is associated with hypertension [27].The objective of this research is the assessment of the subclinical target organ damage of the heart as represented by LV mass and function in patients with essential hypertension and its relation with vitamin D and parathormone hormone.
2. Methods
2.1. Study Population
- The current study has been carried out as an observational retrospective study, enrolling 70 patients who were diagnosed as high hypertensive patients (HTN) with mean age of (50.28±7.8) years. All participants have been selected from the internal medicine and cardiology outpatient clinics and departments at Al-Zahraa University Hospital, Faculty of Medicine (for Girls), Al-Azhar University, during the period from November 2018 to April 2019. All patients were informed of the purpose of the study with written and verbal consent in the presence of a third party according to the ethical guidelines of the ethical committee of Al-Azhar University, Cairo, Egypt.The research did not include patients with structural heart disease, diabetes mellitus, hepatic or renal disease, malignancy, obesity (BMI ≥ 30 kg/m2) and patients with either the vitamin D or calcium supplementation. All patients have a complete history of their demographic data such as age, gender, and duration period with HTN. Moreover, clinical examination and data such as height, weight, and office SBP and DBP were conducted thoroughly.
2.2. Laboratory Investigations
- Six mL of venous blood were withdrawn from each subject, and then centrifuged, and serum was separated and divided into three portions. The first portion used for blood urea, serum creatinine, serum calcium, and lipid profile tests assessment, which were done on Cobas C 311 (Germany) and kits of Roche (Germany). The second portion used for PTH measurement, which was done on immulite 1000 using kits from Siemens (Germany) with (Lot NO. 0356) Reference range: 12-65 ng/L. The third portion was stored at -20 until be used for 25 (OH) vitamin D measurement which was measured by enzyme-linked immunosorbent assay (ELISA) technique using the kit supplied by (Epitop Diagnostics, Inc.) (USA) with Lot NO. VDK007 according to manufacturer instructions with the limit of detection 5.866 ng/ml and sensitivity 4.316 ng/ml. ELISA system used was AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer). ELISA assay employs the quantitative sandwich enzyme immunoassay technique.
2.3. 24-hours of Ambulatory Blood Pressure Monitoring (ABPM)
- The 24-hour use of TONOPORTO GE (software version cardio-soft) was used to start ABPM. The size of the cuff was chosen correctly in relation to the circumference of the arm and applied to the non-dependent arm. Patients were instructed to avoid strong physical exercises and to maintain their arm extended during readings. Every 20 minutes, during the day and every 1hour during the night, the BP estimates were registered.The following parameters were acquired for ambulatory BP surveillance: It was regarded ordinary if the SBP was less than 135 mm Hg and the DBP were less than 85 mm Hg, while normal sleep BP less than 120/70 mm Hg. BP was considered normal. Based on the guidelines of the European Cardiology Society/European Hypertension Society for the Management of Arterial Hypertension [28], a standard total of 24 hours BP was regarded <130/80 mm Hg. Blood pressure variance: Dippers are determined if both SBP and DBP declined by 10 percent or more at night, while non-dipper was defined if SBP or DBP fell by under 10 percent during night. The dipping phenomenon (Nocturnal BP decline) was calculated by the following equation (Awake mean BP-Sleep mean BP)/awake mean BP x 100 [29].
2.4. Standard Transthoracic Echocardiography
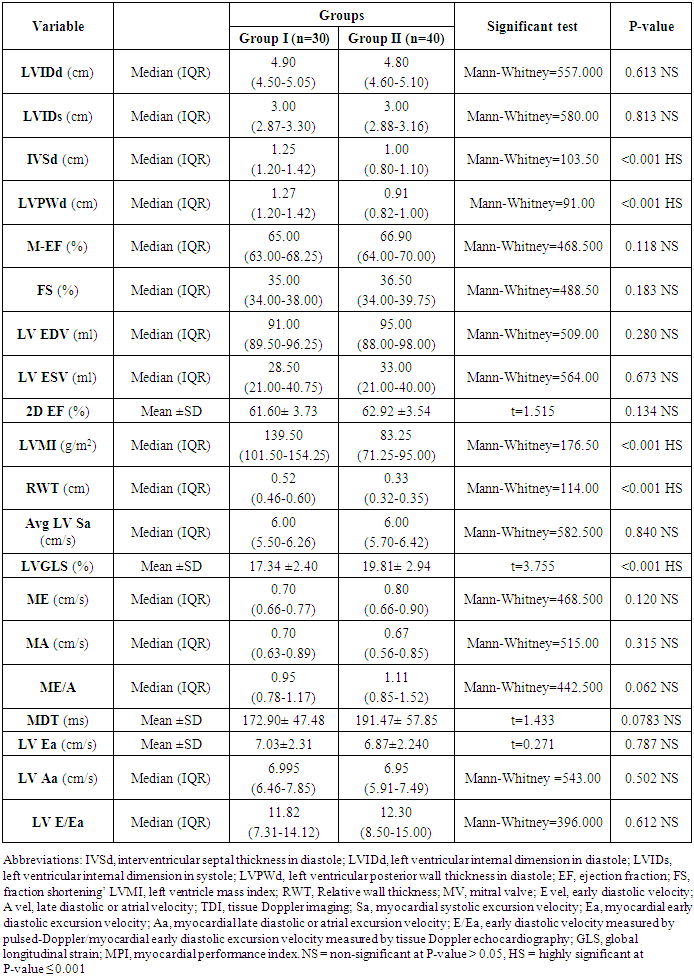
- All patients underwent complete transthoracic echocardiography (TTE) study in both supine and left lateral position [including 2D, M mode, color flow and spectral Doppler as well as tissue Doppler imaging (TDI), strain and 2D speckle tracking (2D-STE)] in the standard views (parasternal long axis, parasternal short axis, apical four, three and two-chamber views) from all accessible windows with ECG physio-signal displayed, with all detected echo-Doppler study. The measurements were made over at least 3 cardiac cycles, and the average value for each parameter was calculated using GE system XD CLEAR 9, Matrix probe M3S multi-frequency 2.5 MHz, with the capability of TDI and grayscale recording for STE. The images were digitally stored for later off-line analysis at echoPac. GE version 210. All parameters were taken According to standards of the American Society of echocardiography, the parameters of LV dimensions and functions including conventional Doppler [mitral valve early diastolic velocity (MV E vel), mitral valve late diastolic velocity (MV A vel), MV E/A ratio and deceleration time (DT)]. LV mass (LVM) was calculated according to the modified Penn formula. LVH was defined by Cornell criteria as the left ventricular mass index (LVMI) >50 gm/m2 in men and >47 gm/m2 in women. The relative wall thickness (RWT) [(IVS or PWT)/LV end-diastolic diameter)] was calculated as an index of the LV geometric pattern (concentric LVH, RWT≥0.45; eccentric LVH, RWT <0.45) [30]. Biplane LV end-diastolic and -systolic volumes were assessed from the apical 2- and 4-chamber images and LV ejection fraction (EF) was calculated using the biplane Simpson's formula.The TDI was obtained from apical 4-chamber and 2-chamber views. The image sector width was set as narrow as possible to allow a frame rate acquisition greater than 90 frames/s. Special attention has been paid to the color Doppler velocity range setting to avoid any aliasing within the image. The mitral annular systolic velocity (Sa) and mitral annular early diastolic velocity (Ea) by pulsed wave tissue Doppler were obtained at septal, lateral, inferior and anterior annular positions then average and E/Ea average ratio was calculated.The LV longitudinal strain was also assessed using 2D STE analysis with QRS onset as the reference point, applying a commercially available strain software package to the LV on echoPac version 210. Images were acquired at 70–90 frames per second at end-expiration in the apical 4-chamber, 3-chamber and 2- chamber views. The Automated Function Imaging software (AFI) was used to track the endocardial contour using a point-to-click approach for three anchor points (two basal and one apical). In every studied case, the LV 2D ST GS percent was automatically obtained.
2.5. Statistical Analysis
- Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS software version 25, Chicago, Illinois).The methods used for statistical analysis were as follows:i. Descriptive statistics:1- Mean Standard deviation (± SD) and range for parametric numerical data, while the Median was used for non-parametric numerical data. Standard deviation is the ideal measure of variability and is usually expressed as plus and minus values (±) to follow the arithmetic mean of the sample.ii. Analytical statistics: 1. Student t-test was used to assess the statistical significance of the difference between the two-study group mean values of quantitative data.2. Mann Whitney Test (U test) was used to assess the statistical significance of the difference between a non-parametric variable between two study groups.3. Chi-Square test was used to examine the relationship between two qualitative variables.4. Pearson and Spearman coefficients of correlation. p-value < 0.05. 0.0015. ROC curve was plotted to define the cut-off value of vitamin D and PTH for prediction of LV systolic dysfunction in patients with HTN.6. P-value: level of significance- P>0.05: Non-significant (NS).- P< 0.05: Significant (S).- P<0.001: Highly significant (HS).
3. Results
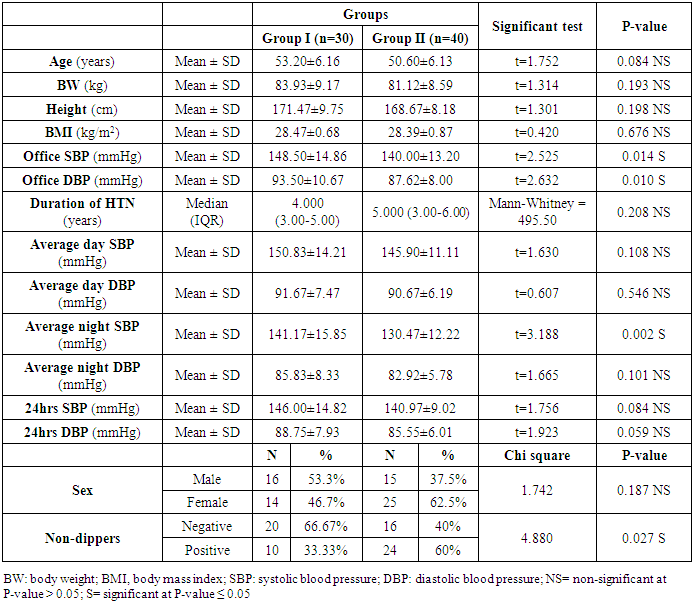
- Seventy hypertensive patients (HTN) were included in this research. They were divided into 2 groups according to the level of vitamin D. The group of patients (I) consisted of 30 patients with vitamin D deficiency (patient group) divided as follows: 16 men and 14 women with a mean age of 53.20±6.16. The second group (II) consisted of 40 hypertensive patients with normal vitamin D (control group) classified as follows, 15 men and 25 women with a mean age of 50.60±6.13.It showed a statistically significant distinction in the range of Office SBP, Office DBP, Average night SBP, and Non-Dipper (P ≤ 0.05) between the group I and group II. While not statistically differences between group I and group II as regard age, BW, height, BMI, duration of HTN, average day SBP, average day DBP, average night DBP, 24hrs SBP, 24hrs DBP and sex (Table 1).
|
|
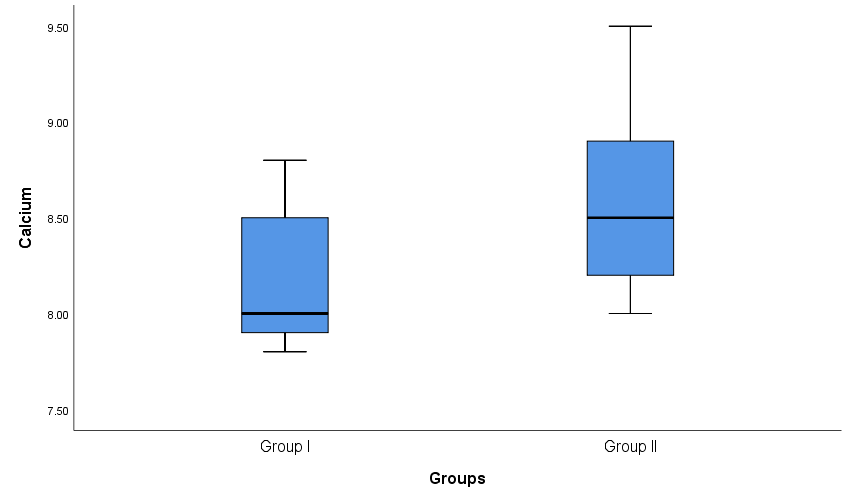 | Figure 1. Box plot show comparison between group I and group II as regard serum calcium |
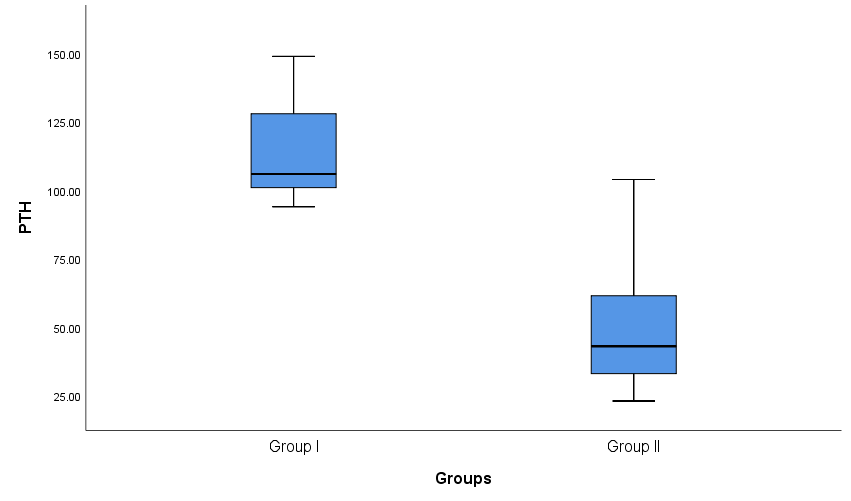 | Figure 2. Box plot show comparison between group I and group II as regard PTH |
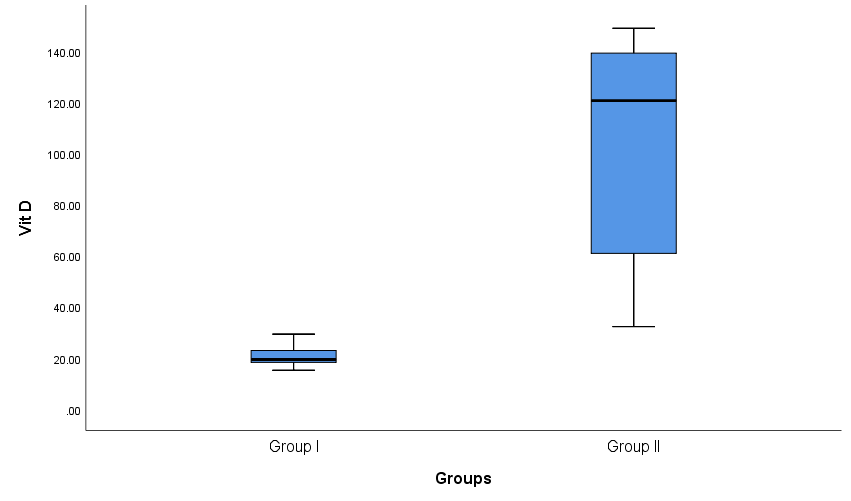 | Figure 3. Box plot show comparison between group I and group II as regard Vit D |
|
|
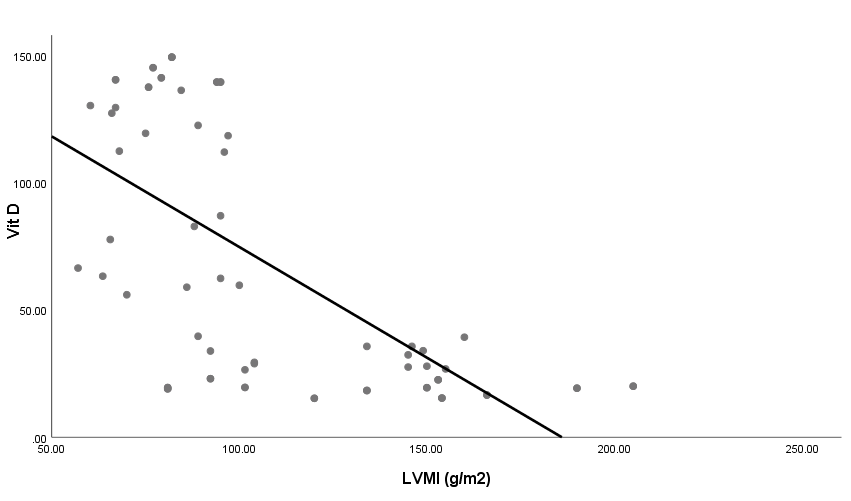 | Figure 4. Linear correlation between LVMI and Vit D |
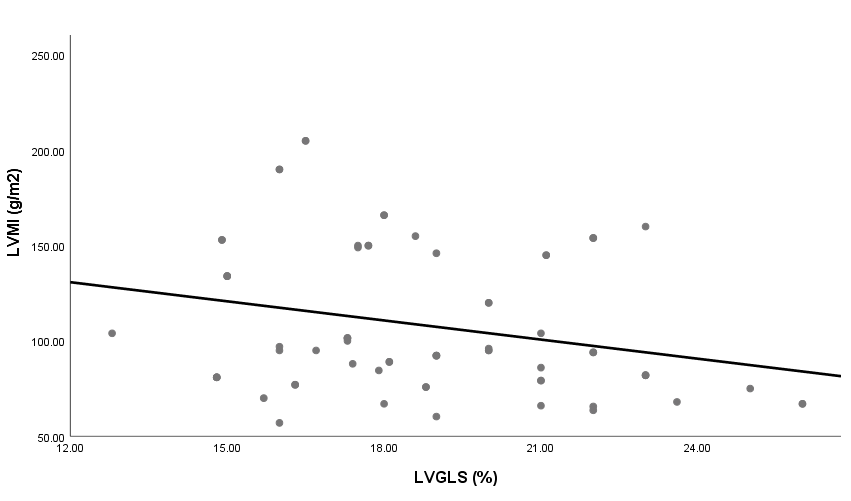 | Figure 5. Linear correlation between LVMI and LVGLS |
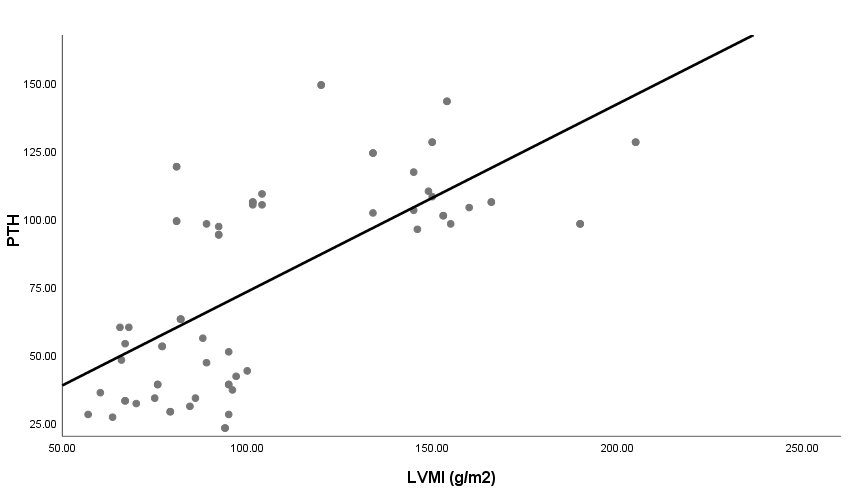 | Figure 6. Linear correlation between LVMI and PTH |
|
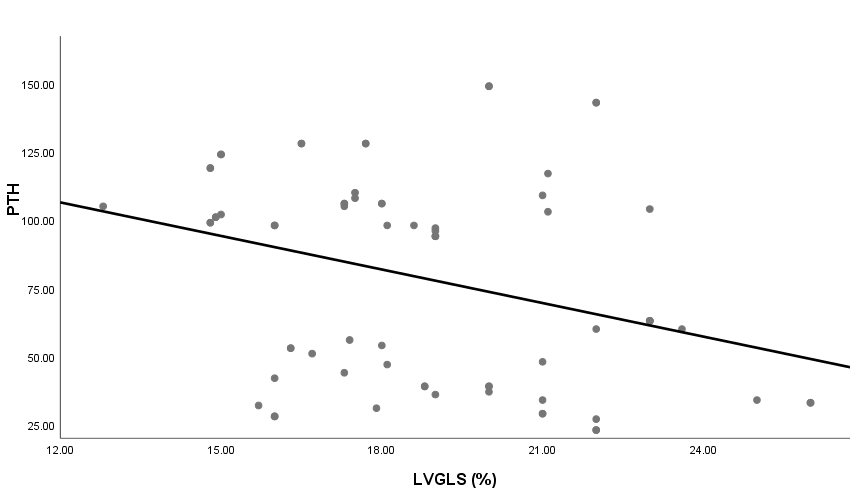 | Figure 7. Linear correlation between LVGLS and PTH |
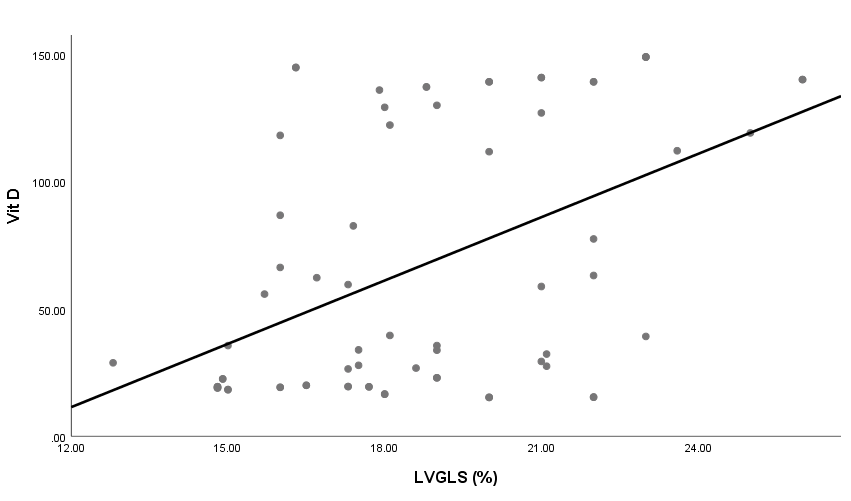 | Figure 8. Linear correlation between LVGLS and Vit D |
|
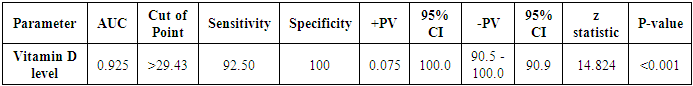
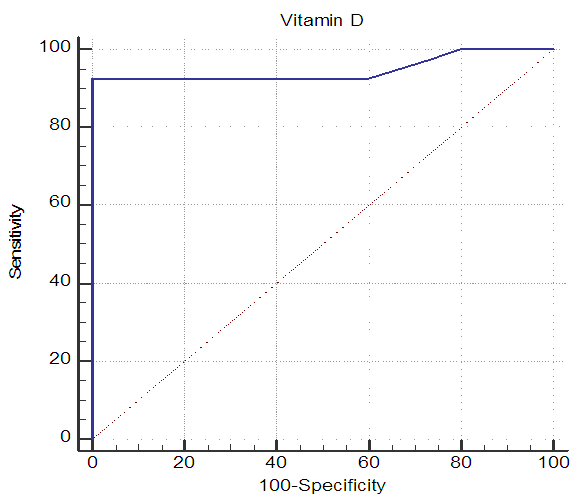 | Figure 9. ROC curve of vitamin D level as a predictor for LV systolic function |
|
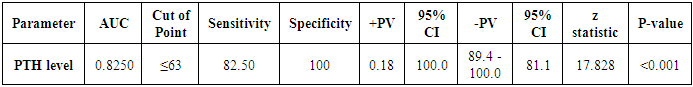
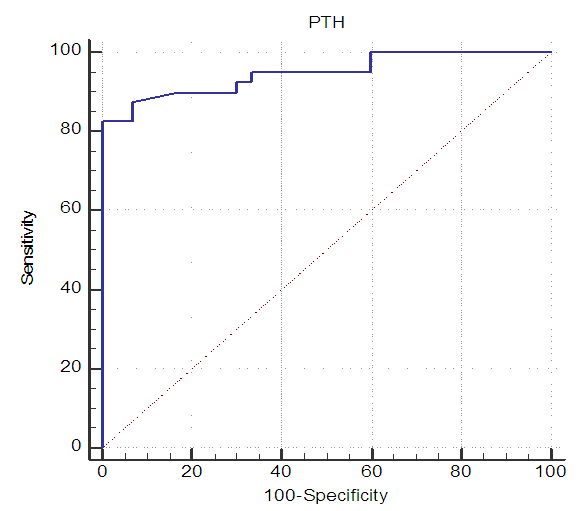 | Figure 10. ROC curve of PTH level as a predictor for LV systolic function |
4. Discussion
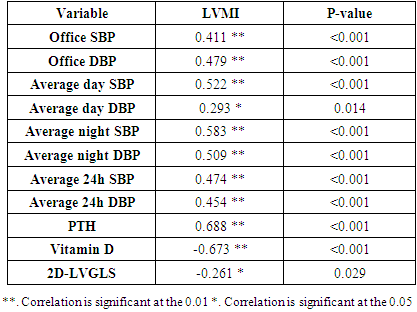
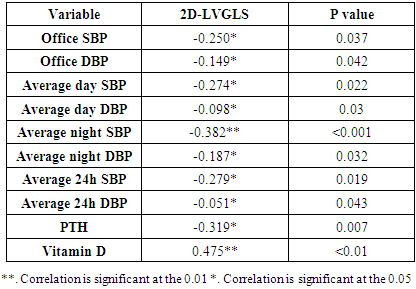
- Blood pressure is a prevalent condition where the artery walls are long-term enough blood to trigger eventual health issues, such as heart illness. High blood pressure is a prevalent condition. Both the quantities of blood the heart pumps and the quantity of blood resistance in the arteries is determined by blood pressure [1]. The greater the blood pressure is, the more blood the heart pumps and the smaller the arteries. Years without any symptoms can lead to high blood pressure (hypertension). Blood vessel and heart damage can continue and be identified even without symptoms [2]. Unchecked high blood pressure raises the risk of severe health issues including cardiovascular. Moreover, the sustained other CV risk variables leading to enhanced CV morbidity and mortality in about 60% of patients globally is considered one of the main complications of hypertension [31]. There are therefore significant challenges to public health in preventing and treating HTN and related target organ damage (TOD) [32].Left ventricular hypertrophy (LVH) is an abnormal increase in left ventricular mass. Which is a marker for and contributes to coronary events, stroke, heart failure, peripheral arterial disease, and cardiovascular mortality in patients with hypertension [33]. Furthermore, it is considered as an adaptive mechanism to maintain or normalize wall stress, sometimes at the expense of diastolic and long-axis systolic function. It is related to changes in LV parameters as chamber dimensions, geometry, and function [34]. These modifications are progressive and eventually can lead to systematic and/or diastolic dysfunctional heart failure (HF) [33].Vitamin D insufficiency is highly prevalent in the general population and presents a serious public health problem [35]. Since both HTN and vitamin D deficiency are highly prevalent worldwide, establishing an association among these two may potentially have wide public health implications but may also be the result of the high prevalence of both conditions rather than a causative link. Even though the majority of recent clinical studies support the hypothesis of an inverse association between the vitamin D serum level and BP, there have been studies that contradict this hypothesis as well [36].The aim of this study is to assess the subclinical TOD of the heart in patients with HTN and its association with vitamin D deficiency and parathormone excess. The study included 30 hypertensive patients with vitamin D deficiency as patients group (G1) and another 40 age, sex and BMI-matched hypertensive patients with normal vitamin D level as the control group (G2). HTN duration was similar in both groups.Left ventricular mass index (LVMI) and RWT (RWT) were considerably greater in G1 than G2, and the LVMI and Vitamin D levels had a very important adverse correlation. The findings of this research coincide with the conclusion of Fallo et al. [37] that vitamin D deficit is independent of enhanced LVM. Such vitamin D deficiency could be linked to the growth of HTN LVH according to the results of Stolarz-Skrzypek et al. [38]. Additionally, both ambulatory blood pressure monitoring (ABPM) readings and percent of non-dippers were significantly higher in G1 than G2. In accordance with Chandana et al. [39] there was also a significantly adverse correlation between the measurements of vitamin D and AMBP and that of the vitamin D serum, and both SBP and SBP are negative. Burgaz et al. [40] also discovered that serum vitamin D levels are inversely linked with HTN in meta-analysis of 18 researches in 2011 [40]. Forman et al. [41] discovered, on the other side, that there was no connection between dietary intakes of vitamin D and supplements and HTN risk. The relationship between vitamin D deficit and HTN may be explained by several mechanisms. Initially, experimental findings have stated that vitamin D participates with a direct suppression of the expression of the renin- angiotensin axis [42]. Second, endothelial and vascular muscle cells express vitamin D receivers and are capable of converting 25-dihydroxyvitamin D into 1.25-dihydroxyvitamin D [43].Putative Vitamin D vascular impacts include smooth proliferation, inflammation, and thrombosis [43]. Vitamin D modules are a broad spectrum. Third, a deficiency of vitamin D causes secondary hyperparathyroidism. Hypertrophy and vascular remodeling are encouraged by PTH [16].Many cases are not aware of the mechanism underlying the non-dipping BP profile. Evidence accumulates that factors associated with volume are often involved. This explains the connection between non-dipping with HTN forms that are salty and sensitive to kidney function, hyperparathyroidism and HTN forms that are induced by mineralocorticoids [44]. Moreover, 24 patients of G1 (80%) showed impaired LV systolic function measured by 2D-LVGLS with significant difference between both groups despite apparently normal EF. Oppositely, G2 patients showed non-significantly more diastolic dysfunction measured by LV E/Ea. Vitamin D has been discovered to have a positive correlation to 2D-LVGLS and vitamin D has been found to be the strongest predictor of LV systolic dysfunction in HTN patients with multivariate assessment. Abdel Rahman et al. [45], found that, depending on serum concentration, vitamin D has a biphasic impact on its cardiac function. Lower vitamin D appears to have a worse systemic function, while greater concentrations appear to be associated with poorer LV diastolic function. By various mechanisms, they clarified their outcomes. Vitamin D deficiency was noted in the manufacturing and deposition of myocardial tissue for induced myocardial hypertrophy and extracellular matrix. The extracellular matrix restructuring can involve progressive LV restructuring, expansion, and HF, mediated by a matrix of metalloproteinases. Vitamin D also has fascinating immunostatic and DNA-protective characteristics at the molecular level. Negative impacts linked to the surplus of vitamin D include hyperphosphatemia, hypercalcemia, enhanced MMP, and medial calcification.Our results detected that 35 hypertensive patients (50%) had increased PTH above normal level and by re-classification of their HTN patients and comparing both groups (G A and G B), it has found a higher IVSd, LVPWd thickness, LVMI, RWT and lower 2D-LVGLS in G A compared to G B. Our results were in agreement with Näppi et al. [46] who concluded that hyper-parathyroidism induces LVH and slight impairment of LV systolic function measured by EF. LV systolic function indicators were used and assessed with 2D-LVGLS, because an assessment of STE imaging could determine the deformation and subtract the impact of tethering and translation movement of the whole heart and therefore, it can be used with more vital function than echocardiographic measures [47]. Renin disturbance-angiotensin-aldosterone system and structural and functional alterations of the vascular wall have been reported to have related hyper-parathyrodism to HTN. An increased prevalence has been observed of heart structural abnormalities, such as LVH, valvular and myocardial calcification. Associations between PTH and LVH, LVH and serum calcium were found [48].
5. Conclusions
- Subclinical target organ damage is significantly higher in hypertensive patients with vitamin D deficiency than hypertensive patients with normal vitamin D levels. Patients with vitamin D deficiency or excess PTH have higher LVMI, higher AMBP readings, a higher percent of non-dippers, and more impaired LV systolic function. Serum vitamin D<29 ng/mL is a strong predictor of LV systolic dysfunction in patients with essential HTN.
Limitations
- This study has small sample size with smaller sub-groups numbers so a larger study is recommended. Also, we didn`t study the effect of vitamin D as anti-hypertensive treatment on different group patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML