Nalgaa Abou-Elfattah Tawfik1, Abeer Mohammed Abdul-Mohymen2, Marwa Mohsen Ramadan3, Asmaa M. El-Nasser4
1Department of Internal Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
3Department of Community and Occupational Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
4Department of Medical Microbiology and Immunology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Nalgaa Abou-Elfattah Tawfik, Department of Internal Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Serum cartilage oligomeric matrix protein (COMP) is a non-collagen glycoprotein produced by the cartilage, synovium, tendon, and meniscus. COMP is a reliable factor for monitoring cartilage damage. COMP has been studied as a biological marker for diagnostic and prognostic purposes. Musculoskeletal ultrasound (MSUS) imaging technology have allowed imaging of inflamed joints in rheumatoid arthritis patients. Objective: To assess the relationship between serum COMP levels and the articular cartilage damage based on sonographic knee cartilage thickness (KCT), disease activity, and other inflammatory markers in RA patients. Patients and Methods: Ninty participants were divided into two groups. Group I: Forty-five rheumatoid arthritis patients, which were subdivided according to disease activity into mild, moderate and sever, carried out from rheumatology outpatient clinic and inpatient in the Internal Medicine Department, Al-Zahraa University Hospital, all patients were female with their age ranging from 18 to 70 years with mean ± SD was (44.4±12.03). Group II: Forty-five age and sex-matched as control were included. Serum COMP level, Anti-Cyclic Citrullinated Peptides (ACCP), Rheumatoid Factor (RF), High Sensitive C- Reactive Protein (hs-CRP), were assayed. Also, sonographic knee cartilage thickness and Disease activity score (DAS28) were measured. Results: There were a highly significant increase the COMP level in the patient groups (the median is 301, and the interquartile range is 48-7954.9) in comparison to the control group (the median is 91.3, and the interquartile range is 39-146) with a P-value of 0.000, and highly significant increase the COMP level in the patient groups according to disease activity. There was a highly significant reduction of the knee cartilage thickness regarding lateral condyle (LC), medial condyle (MC) and intercondylar notch (IN) bilaterally compared with the control group. There were highly significant negative correlations between COMP and RKCTLC, RKCTIC and LKCTIC (P= 0.000), significant negative correlations between COMP and RKCTMC (P= 0.004), LKCTMC (P= 0.01), while there was no significant correlation between COMP and LKCTLC (P= 0.07), the mean of the right side was (P= 0.09) and the mean of the left side was (P= 0.22). Also, significant positive correlations between COMP and DAS28 (P= 0.03) was found. Conclusion: COMP can be used as a diagnostic biomarker for RA, based on its significant correlations with disease activity, ACCP, and knee cartilage thickness.
Keywords:
Rheumatoid arthritis, Cartilage oligomeric matrix protein (COMP), Anti-CCP antibody, DAS28, Knee cartilage thickness (KCT)
Cite this paper: Nalgaa Abou-Elfattah Tawfik, Abeer Mohammed Abdul-Mohymen, Marwa Mohsen Ramadan, Asmaa M. El-Nasser, Serum Cartilage Oligomeric Matrix Protein (COMP) a Biomarker of Rheumatoid Arthritis, Disease Activity and Knee Cartilage Thickness, International Journal of Internal Medicine, Vol. 8 No. 2, 2019, pp. 25-35. doi: 10.5923/j.ijim.20190802.03.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease [1] characterized by pain in joints and destruction of bone and cartilage [2]. Joint destruction in RA results from the invasion of the cartilage and subchondral bone by the hyperplastic synovium, with synovial fibroblasts and inflammatory cells [3]. Irreversible joint destruction is associated with significant impairment of functional capacity of life [4]. Diagnosis of RA depends mainly on clinical manifestations of the disease and the presence of several diagnostic markers [5]. Numerous serological markers of RA have been described [6], the most commonly used markers are the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and rheumatoid factor (RF) [7]. Anti-cyclic citrullinated peptide (CCP) is one of the markers that have been used for the diagnosis of RA [8]. New markers of joint destruction of RA patients and the development of progressive joint damage, which are found in serum and synovial fluid might help monitor the course of RA [9]. Cartilage oligomeric matrix protein (COMP) is an original molecular marker of cartilage destruction [10]. COMP is a non -collagen glycoprotein produced by the cartilage, synovium, tendon, and meniscus [11]. It is a member of the thrombospondin5 glycoprotein family; it is a 524 kDa, extracellular protein [2]. COMP has five identical subunits, each subunit consists of 755 amino acids, which is considered a diagnostic and prognostic indicator and used as a marker of the disease severity, and recovery with treatment in RA [12]. On the other hand, the evaluation of articular cartilage damage, especially at the early stages, an ultrasound scan has a higher sensitivity than plain radiographs [13]. This study aimed to assess the relationship between serum COMP levels and the articular cartilage damage based on sonographic knee cartilage thickness (KCT), disease activity, and other inflammatory markers in patients.
2. Patients and Methods
This study was an observational analytical case-control study. The sample size was calculated by using Power and Sample size program software (PS). We have planned the study of matched sets of cases and controls with 1 matched control per case. We expect the true odds ratio for rheumatoid arthritis in exposed subjects relative to unexposed subjects is 6; we needed to study 45 case-patients with 1 matched control per case to be able to reject the null hypothesis that this odds ratio equals 1 with probability (power) 0.8. The Type I error probability associated with this test of this null hypothesis is 0.05.So, this study was conducted on ninety participants, which were divided into two groups. Group I: forty-five rheumatoid arthritis patients were taken from rheumatology outpatient clinic and inpatient Internal Medicine Department, Al-Zahra University hospital, in the period between September 2018 to January 2019, after taking written consent from all patients and controls regarding the ethical committee of the university. Patients diagnosed according to the 2010 ACR/EULAR RA classification criteria.Participants were subdivided according to disease activity into three groups each group included fifteen patients. Group Ia: patients with mild disease activity, whose DAS28 score <3.2. Group Ib: patients with moderate disease activity whose DAS28 score ≥3.2 and <5.1. Group Ic: patients with severe disease activity whose DAS28 score ≥5.1. Group II: forty-five healthy volunteers served as a control group with matched age and sex. Inclusion criteria: Adult female rheumatoid arthritis patients. Exclusion criteria: other autoimmune diseases such as systemic lupus erythematosus, overlapping syndrome, pregnant or nursing patients, hyperuricemia, and patient under biological treatment.All patients and control participants had the following clinical examination, laboratory investigations, and radiological imaging, which include:1. Full medical history and thorough clinical examination were done for all patients and control subjects, complete musculoskeletal examination, DAS28 assessment was done for all patients.2. Laboratory investigations include complete blood cell count (CBC), erythrocyte sedimentation rate (ESR), serum total cholesterol (TC), serum triglyceride (TG), serum calcium, serum phosphorus, Rheumatoid factor (RF), High Sensitive C- Reactive Protein (hs-CRP), Anti-Cyclic Citrullinated Peptides (ACCP) and Cartilage Oligomeric Matrix Protein (COMP).Seven ml of peripheral venous blood were withdrawn from each individual and divided into three aliquots; 2 ml were collected in an EDTA tube for CBC analysis, 0.8 ml were collected in citrate tube for ESR measurement. The remaining part was collected in serum separator tube, centrifuged at 3500 rpm for 10 min and serum was divided into three portions; the first one was used for measurement of total cholesterol, triglyceride, calcium, and phosphorus; the second part was used for Rheumatoid factor measurement, and the third part was frozen at -20°C for later analysis of High Sensitive C- Reactive protein, Anti-Cyclic Citrullinated Peptides and Cartilage Oligomeric Matrix Protein (COMP).Complete blood count was determined by cell counter Sysmex KX21N (Germany) using kits of siemens (Germany). Total cholesterol, triglyceride, calcium, and phosphorus were done using Cobas C311 (Germany) and kits of Roche (Germany).Rheumatoid factor (RF) was measured by semi-quantitative latex agglutination kit supplied from Omega Diagnostics, Scotland, United Kingdom, A vitex RF (Ref. OD063), negative results are at an RF concentrate on below 8 U/L, and positive results are at an RF concentration above 8 U/L. High Sensitive C-Reactive Protein (hs-CRP) was measured by quantitative solid-phase enzyme-linked immunosorbent assay (ELISA) kit supplied from BioScience, San Francisco, USA, Cat. No. 10603 (Sensitivity: 1 ng normal expected values in adult serum: 68-8200 ng/ml); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer). Anti-Cyclic Citrullinated Peptide 3 (ACCP3) was measured by semi-quantitative ELISA for the detection of IgG anti-CCP3 antibodies in patient sera (using QUANTA Lite CCP3 IgG ELIZA kit from Inova Diagnostics, San Diego, USA) (Ref. No.704535); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer) Negative results are at a concentration below 20 U/mL, positive results are at a concentration above 20 U/mL (weak positive 20-39, moderate positive 40-59, strong positive equal or above 60 U/mL).Cartilage Oligomeric Matrix Protein (COMP) was measured by quantitative double-antibody sandwich ELISA kit supplied from Bioassay Technology Laboratory, China, Cat. No. E1486Hu (Standard curve range: 30-9000 pg/ml and sensitivity: 15.66 pg/ml); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer).3. Radiological investigations:B mode and Doppler MSUS (color and power Doppler) diagnostic examination was don at knee cartilage thickness (KCT), in three-position bilaterally, lateral condyle (LC), medial condyle (MC) and intercondylar notch (IN).Statistical analysis:Comparison of numerical variables between 2 groups was done using Student’s t-test for normally distributed data and the Mann Whitney U test for nonparametric one. Comparison of numerical variables between more than two groups was done using ANOVA for normally distributed data while the Kruskal Wallis test for nonparametric one. Correlation between various variables was done using Pearson and Spearman rank correlation equation for normal and non-normal variables, respectively. All statistical calculations were done using computer programs SPSS version 23. Mean, standard deviations and ranges were used to describe quantitative variables, while median and interquartile ranges (25%-75%) were used for non-parametric data. The Student’s independent t-test was used for the differences between means of two continuous variables while Man Whitney U test was used for non-parametric data. P-value was taken at the predetermined threshold probability, the significance level of 0.05 and 95% confidence limit. The results were deemed to be statistically significant if the P-value was <0.05.
3. Results
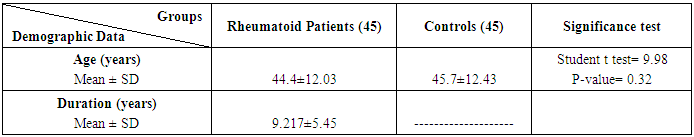
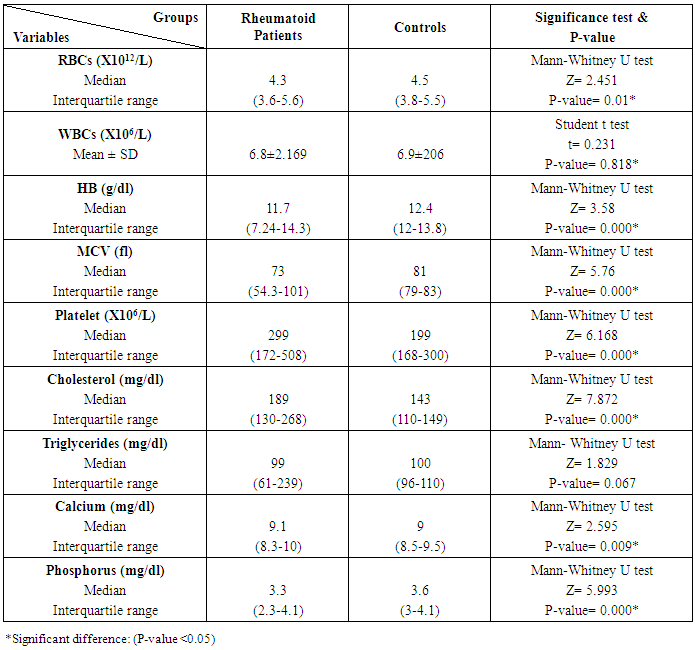
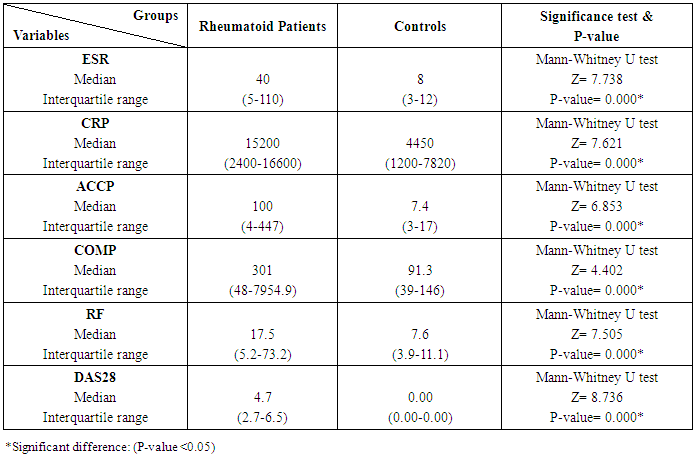
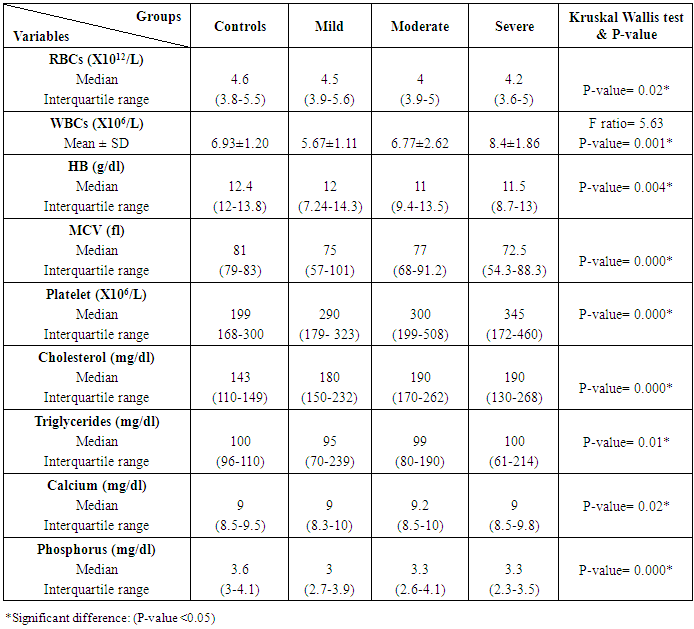
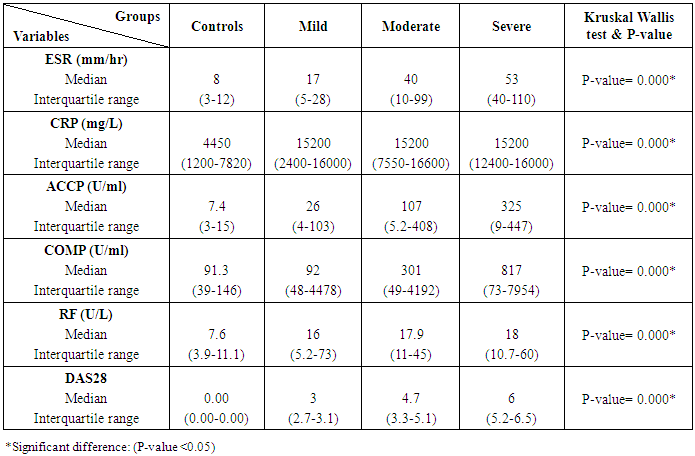
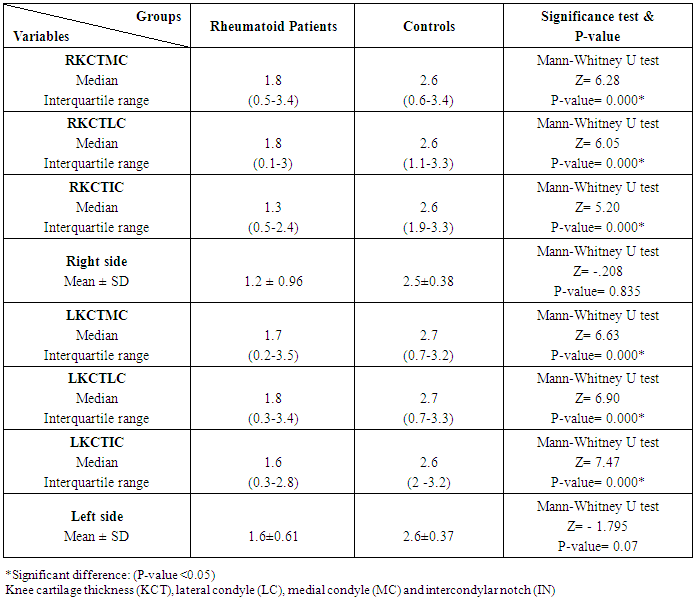
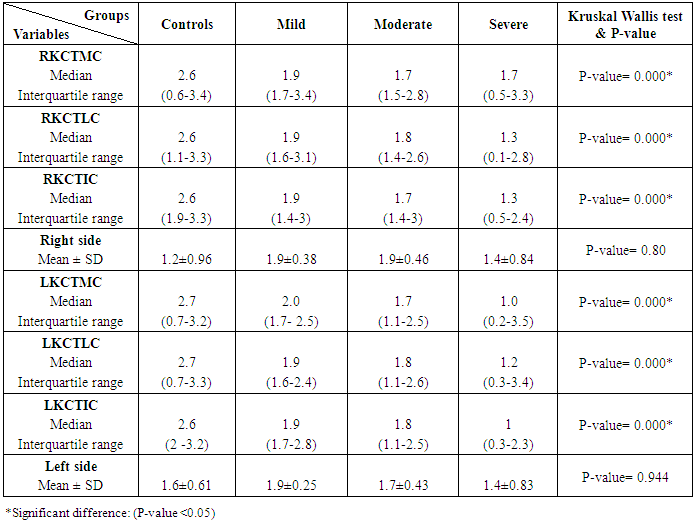
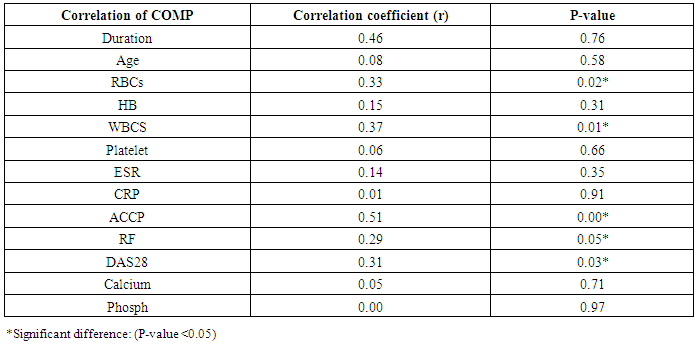
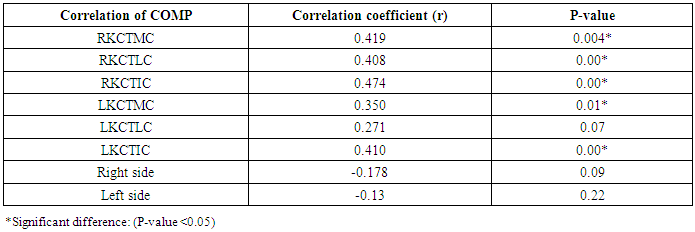
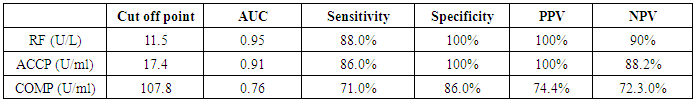
The study included 45 RA patients and 45 healthy controls. The RA patients were divided according to the disease severity into mild, moderate, and severe. The mean ± SD of age (44.4±12.03 and 45.7±12.43 for both patients and controls, respectively) with no significance difference between patient and control groups (P= 0.32). The mean ± SD of the disease duration in years among patients were (9.217±5.45) (Table 1). There was a significant decrease of RBCs count in the patient group than in the control group with a P-value of 0.01, a highly significant decrease of Hb, MCV, serum phosphorus concentration in the patient group than in the control group with a P-value of 0.000, a highly significant increase of the platelet count, serum cholesterol, in the patient group than in the control group with a P-value of 0.000, a significant increase of the serum calcium level in the patient group than in the control group with a P-value of 0.009, while there was no significant difference of WBCs count (P= 0.818), serum triglycerides level (P= 0.067), between the patient and the control groups (Table 2). There was a highly significant increase of all the inflammatory markers ESR, CRP, ACCP, RF, and COMP in the patient group than in the control group; with a P-value of 0.000. Also, DAS28 was highly significantly high in the patient group than in the control group with a P-value of 0.000 (Table 3 & Figure 1).The comparison between laboratory tests and the severity of the disease mild, moderate, and severe cases is demonstrated in the table (4).In the comparison between the disease severity and inflammatory markers, there was the highly significant positive difference between the disease severity and all of the inflammatory markers and DAS28 (P= 0.000) (Table 5). In the comparison between knee cartilage thickness among rheumatoid patients and controls, There were a highly significant reduction of the knee cartilage regarding RKCTMC, RKCTLC, RKCTIC, LKCTMC, LKCTLC and LKCTIC in the patient group than in the control group with a P-value of 0.000, while there were no significant difference regarding the overall knee cartilage in the mean Right side between the patient group (the mean ± SD is 1.2±0.96) and the control group (the mean ± SD is 2.5±0.38) with a P-value of 0.835 or mean left side between the patient group (the mean ± SD is 1.6±0.61) and the control group (the mean ± SD is 2.6±0.37) with a P-value of 0.07 (Table 6). In the comparison between the disease severity and knee cartilage thickness, there were highly significant difference between the disease severity and the knee cartilage regarding RKCTMC, RKCTLC, RKCTIC, LKCTMC, LKCTLC and LKCTIC (P= 0.000), while there was no significant difference regarding mean ± SD of the overall Right (P= 0.80) and left side (P= 0.944) knee cartilage thickness (Table 7).In the correlation of COMP with different studied parameters among rheumatoid patients, there were a highly significant positive correlation between COMP and ACCP (P= 0.000), significant positive correlations between COMP and WBCs count (P= 0.01), and DAS28 (P= 0.03), RF (P= 0.05) a significant negative correlations between COMP and RBCs count (P= 0.02), while there was no significant correlation between COMP and the duration of the disease (P= 0.76), age of patients (P= 0.58), Hb concentration (P= 0.31), platelet count (P= 0.66), CRP (P=0.91), ESR (P= 0.35) serum calcium level (P= 0.71) or serum phosphorus level (P= 0.97) (Table 8).In the correlation of COMP with knee cartilages thickness, there were highly significant negative correlations between COMP and RKCTLC, RKCTIC and LKCTIC (P= 0.000), significant negative correlations between COMP and RKCTMC (P= 0.004), LKCTMC (P= 0.01), while there was no significant correlation between COMP and LKCTLC (P= 0.07), the mean of the right side (P= 0.09) or the mean of the left side (P = 0.22) (Table 9). The cut-off point of RF for the diagnosis of rheumatoid arthritis was 11.5 with a sensitivity of 88.0% and specificity of 100.0% PPV of 100% and NPV of 90%. The cut-off point of ACCP for the diagnosis of rheumatoid arthritis was 17.4 with a sensitivity of 86.0% and specificity of 100% PPV of 100% and NPV of 88.2%. The cut-off point of COMP for the diagnosis of rheumatoid arthritis was 107.8 with a sensitivity of 71.0% and specificity of 86.0% PPV of 74.4% and NPV of 72.3% (Table 10 and Figures 2 & 3).Table 1. Demographic data of rheumatoid patients and control
 |
| |
|
Table 2. Laboratory tests among rheumatoid patients and control
 |
| |
|
Table 3. Inflammatory markers among rheumatoid patients and control
 |
| |
|
Table 4. Laboratory tests among rheumatoid patients' groups and control
 |
| |
|
Table 5. Inflammatory markers among rheumatoid patients' groups and control
 |
| |
|
Table 6. Knee cartilage thickness among rheumatoid patients and controls
 |
| |
|
Table 7. Knee cartilage thickness among rheumatoid patients' groups and control
 |
| |
|
Table 8. Correlation of COMP with different studied parameters among rheumatoid patients
 |
| |
|
Table 9. Correlation of COMP with different Knee cartilage thickness among rheumatoid patients
 |
| |
|
Table 10. Diagnostic accuracy of inflammatory marker in the diagnosis of rheumatoid arthritis
 |
| |
|
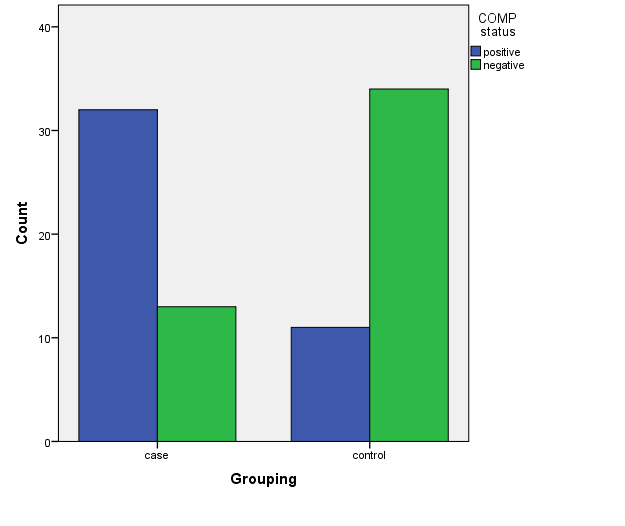 | Figure 1. COMP level in the patient and control group |
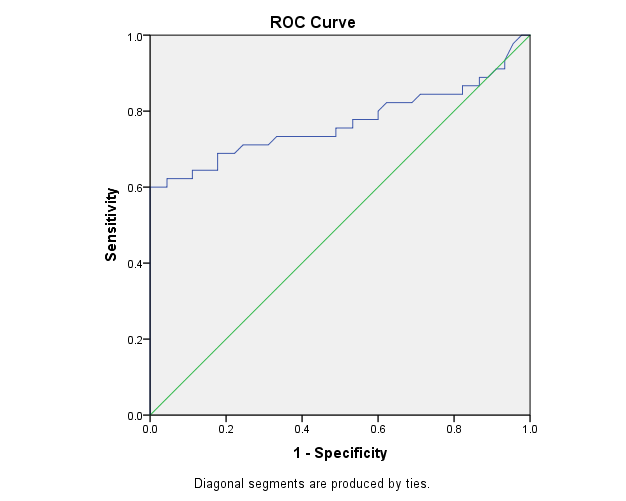 | Figure 2. The ROC curve analysis of COMP in the detection of rheumatoid arthritis cases |
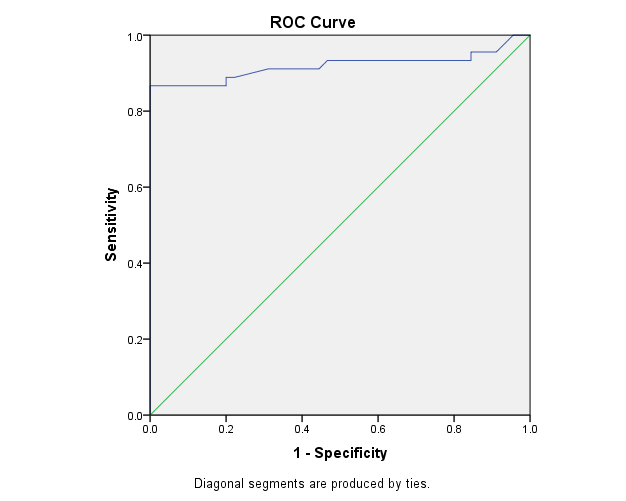 | Figure 3. The ROC curve analysis of ACCP in the detection of rheumatoid arthritis cases |
4. Discussion
Rheumatoid arthritis is the most common chronic inflammatory arthritis that affects the synovial joints, cartilage destruction, and bony erosions [14]. The most significant characteristic of RA is proliferative and inflammatory synovitis of the peripheral joint [15]. COMP is a marker of cartilage degradation, which was found in serum and synovial fluid of RA patients [16]. Inflammatory synovium has been considered as a potential tissue source of COMP since the molecule has been detected in the synovium [15]. COMP level is significantly higher among RA patients compared to control subjects; production of COMP is associated with duration of erosive joint damage in RA [10]. Ultrasound is used to measure knee cartilage thickness to assess joint damage with an inverse relationship with serum COMP level [17].In the present study there was a statistically significant elevation in serum COMP level in the RA patients group compared to the control group (P-value= 0.000), in agreement with Sweilam et al. [2] and also in agreement with Sakthiswary et al. [13], El Defrawy et al. [17], Algergawy et al. [18] and Liu et al. [15]. The synovial membrane is an essential source of COMP. The increased COMP in RA patients could be due to the osteoarthritis and the synovial membrane inflammation [19]. In the current study, COMP levels were significantly higher in patients with more active disease, these results are in agreement with Liu et al. [15], Hussein et al. [20], and Andrade et al. [21] who showed similar results as RA patients with severe joint damage had higher serum COMP. The increase in turnover of cartilage matrix in joint inflammation may explain that correlation. Our study showed no significant correlation between COMP levels and age of the patients or duration, in agreement with Hussein et al. [20], and disagreement with Wislowska and Jablonska [22] and El Defrawy et al. [17] they found COMP levels significantly correlated with the age of RA patients. RA is a very heterogeneous, and the variation in the criteria of diagnosis and patients' characteristics in these studies might explain the different findings [13].In the present study, there was a significant correlation between COMP levels and DAS28, this result agrees with Sweilam et al. [2], Saghafi et al. [10], Sakthiswary et al. [13] and Andersson et al. [23]. Also, in our study, there were significant correlations between COMP levels and ACCP, RF in RA Patients in agreement with Sweilam et al. [2]. This can be explained by the increased fragmentation of the affected joints and the release of these molecules into the circulation in active disease [23]. Knee cartilage thickness using ultrasound was used to assess joint damage, which showed an inverse relationship with serum COMP level, Sakthiswary et al. [13]. This result is in agreement with our results, we found a significant decrease in knee cartilage thickness of patients in comparison to healthy control, and in patients of different degrees of disease activity groups mild, moderate and severe. Also, there is a significant correlation between knee cartilage thickness and serum COMP. This result also agrees with Saghafi et al. [10]. Andersson et al. [23] found that a high level of COMP is associated with cartilage and joint destruction in RA patients. Synovial inflammation in RA causes loss of components of the cartilage matrix by the enzymatic degradation mediated by cytokines of inflammation; one of these components is the COMP [13].In the present study sensitivity of COMP was 71% and specificity was 86%, this result is in agreement with Sweilam et al. [2] who reported that the sensitivity of COMP was 74% and the specificity was 83.33%, also comparable with Saghafi et al. [10], who reported COMP sensitivity of 68% and specificity of 72%., also this finding were in concordance by Liu et al. [15] which found that COMP specificity was 88.2% and sensitivity was 81.7%.
5. Conclusions
COMP can be used as a diagnostic biomarker for RA, which significantly correlates with disease activity, ACCP, and knee cartilage thickness and can be used to predict patients with a high risk of rapidly developing destruction of knee cartilage.
References
| [1] | Aref M and Ahmed H (2015). Cartilage oligomeric matrix protein as new marker in diagnosis of rheumatoid arthritis. Mod Chem appl., 3(151): 2. |
| [2] | Sweilam MA, Attia MA, El-Saadany HM and Ibrahim AS (2018). Study the role of serum cartilage oligomeric matrix protein (COMP) in the diagnosis of rheumatoid arthritis patients. The Egyptian Journal of Hospital Medicine, 73(11): 8061-8073. |
| [3] | Distler JH, Jüngel A, Huber LC, Seemayer CA, Reich CF 3rd, Gay RE, et al. (2005). The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci U S A, 102(8): 2892-2897. |
| [4] | Wan SW, He HG, Mak A, Lahiri M, Luo N, Cheung PP and Wang W (2016). Health-related quality of life and its predictors among patients with rheumatoid arthritis. Appl Nurs Res, 30: 176-183. |
| [5] | Wagner E, Skoumal M, Bayer PM and Klaushofer K (2009). Antibody against mutated citrullinated vimentin: a new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol Int., 29(11): 1315-1321. |
| [6] | Liu X, Jia R, Zhao J and Li Z (2009). The role of anti-mutated citrullinated vimentin antibodies in the diagnosis of early rheumatoid arthritis. J Rheumatol, 36(6): 1136-1142. |
| [7] | Hobbs KF and Cohen MD (2012). Rheumatoid arthritis disease measurement: a new old idea. Rheumatology (Oxford), Suppl 6: vi21-7. |
| [8] | Abdul Wahab A, Mohammad M, Rahman MM and Mohamed Said MS (2013). Anti-cyclic citrullinated peptide antibody is a good indicator for the diagnosis of rheumatoid arthritis. Pak J Med Sci, 29(3): 773-777. |
| [9] | Krabbe S, Bolce R, Brahe CH, Døhn UM, Ejbjerg BJ, Hetland M, et al. (2017). Investigation of a multi-biomarker disease activity score in rheumatoid arthritis by comparison with magnetic resonance imaging, computed tomography, ultrasonography, and radiography parameters of inflammation and damage. Scand J Rheumatol, 46(5): 353-358. |
| [10] | Saghafi M, Khodashahi M, Saadati N, Azarian A, Rezaieyazdi Z, Salehi M and Sahebari M (2017). Relationship between cartilage oligomeric matrix protein (COMP) and rheumatoid arthritis severity. Electron Physician, 9(12): 5940-5947. |
| [11] | Robinson WH, Lindstrom TM, Cheung RK and Sokolove J (2013). Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol, 9(5): 267-276. |
| [12] | Tseng S, Reddi AH and Di Cesare PE (2009). Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights, 4: 33-44. |
| [13] | Sakthiswary R, Rajalingam S, Hussein H, Sridharan R and Asrul AW (2017). Cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and its correlation with sonographic knee cartilage thickness and disease activity. Clin Rheumatol, (12): 2683-2688. |
| [14] | Firestein GS, Budd RC, Gabriel SE and McInnes IB (2017). Etiology and pathogenesis of rheumatoid arthritis. Kelley and Firestein's textbook of rheumatology (10th ed), Saunders/Elsevier, Philadelphia, USA, pp. 1115-1166 Chapter 69. |
| [15] | Liu F, Wang X, Zhang X, Ren C and Xin J (2016). Role of Serum cartilage oligomeric matrix protein (COMP) in the diagnosis of rheumatoid arthritis (RA): A case-control study. J Int Med Res, 44(4): 940-949. |
| [16] | Mc Ardle A, Flatley B, Pennington SR and FitzGerald O (2015). Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther, 17(1): 141. |
| [17] | El Defrawy AO, Gheita TA, Raslan HM, El Ansary MM and El Awar AH (2016). Serum and synovial cartilage oligomeric matrix protein levels in early and established rheumatoid arthritis. Z Rheumatol, 75(9): 917-923. |
| [18] | Algergawy SA, Abd El-Sabour M, Osman AS, Emam SM and Elham N (2013). Early diagnostic and prognostic values of anti-cyclic citrullinated peptide antibody and cartilage oligomeric matrix protein in rheumatoid arthritis. Egypt J Immunol, 20(2): 11-20. |
| [19] | Hassan G, Aboelnour E, Ibrahim O and Hohammed EF (2016). Novel molecular diagnostic marker in the evaluation of cartilage destruction in patients with rheumatoid arthritis. Journal of Current Medical Research and Practice, 1(3): 72-78. |
| [20] | Hussein DA, El Bakry SA, Morshedy NA and Ibrahim SE (2018). Role of cartilage oligomeric matrix protein (COMP) as a prognostic biomarker in follow-up of early rheumatoid arthritis patients: Correlation to musculoskeletal ultrasonographic findings. The Egyptian Rheumatologist, 40(4): 221-226. |
| [21] | Andrade FD1, Bender AL, da Silveira IG, Stein H, von Mühlen CA and Staub HL (2009). Cartilage oligomeric matrix protein/thrombospondin-5 (COMP/TSP-5) levels do not correlate to functional class in patients with rheumatoid arthritis. Clin Rheumatol, 28(12): 1441-1442. |
| [22] | Wisłowska M and Jabłońska B (2005). Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatol, 24(3): 278-284. |
| [23] | Andersson ML1, Svensson B, Petersson IF, Hafström I, Albertsson K, Forslind K, et al. (2013). Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord, 14: 229. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML








