-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2019; 8(1): 6-10
doi:10.5923/j.ijim.20190801.02

Effect of Prolonged Injection of Kisspeptin-54 on Hypothalamo-Pituitary-Gonadal Axis in Adult Male Rats
1Faculty of Medicine, Department of Physiology, Zagazig-University, Egypt
2College of Public Health and Health Informatics, Qassim University, Saudi Arabia
Correspondence to: Husam M. Edrees, Faculty of Medicine, Department of Physiology, Zagazig-University, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Kisspeptin-54 is a neuropeptide encoded with Kiss-1 gene which signal by G protein receptors. It has an essential role in controlling reproductive axis by inducing hypothalamic gonadotropin-releasing hormone which in turn induce adenohypophyseal gonadotropins with subsequent stimulation of sex hormones and gamete development from the gonads. Aim: to investigate the effects of prolonged continuous peripheral subcutaneous injection of kisspeptin-54 by use of Alzet miniosmoticpumps on blood levels of LH, FSH, testosterone, testis weight, count and motility of sperms in adult male rats. Material and Methods: two groups of adult male rats (ten rats for each group) were used in the experiments to investigate the results of subcutaneous administration of kisspeptin-54 for 13 days using Alzet miniosmoticpumps implanted subcutaneously in the interscapular region to guarantee continuous delivery of Kisspeptin-54 throughout the study (50 nmol/day). Results: the study results showed that prolonged injection of kisspeptin-54 at a dose of 50 nmol/day for thirteen days in male rats decreased significantly blood level of LH, FSH, testosterone (P < 0.001) and testis weight, sperm count and motility (P < 0.001). Conclusion: it can be concluded that chronic, prolonged and continuous subcutaneous administration of Kisspeptin-54 may affect male fertility by causing significant decrease in testicular weight and sperm viability, count and motility through direct desensitization of hypothalamic–pituitary–gonadal axis rather than direct inhibitory effect on the gonads.
Keywords: Kisspeptin-54, LH, FSH, Testosterone, Alzet minipumps
Cite this paper: Husam M. Edrees, Effect of Prolonged Injection of Kisspeptin-54 on Hypothalamo-Pituitary-Gonadal Axis in Adult Male Rats, International Journal of Internal Medicine, Vol. 8 No. 1, 2019, pp. 6-10. doi: 10.5923/j.ijim.20190801.02.
Article Outline
1. Introduction
- Infertility is an irritating health problem with a rising ratio in the world, so, a serious need to improve innovative therapeutic ways for treating infertility. Kisspeptin has a significant regulatory effect on reproductive axis. Reproductive activities are stimulated by hypothalamic gonadotropin-releasing hormone (GnRH) which subsequently stimulate the adenohypophyseal gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) which induce the release of sex hormones and gamete development from the gonads [1]. The kisspeptins belong to arginine-phenylalanine (RF) amides which have been discovered to have a therapeutic role in treating disorders of reproduction. The endogenous ligand of kisspeptin-54 receptors (G protein-coupled receptors) is discovered to be RF amides peptides [2]. Kisspeptins have an important effect on puberty and regulation of productivity by inducing secretion of hypothalamic gonadotrophin releasing hormone (GnRH) aided by the neuropeptides neurokinin B and dynorphin [3]. Recent researches proofed that kisspeptin has a direct effect on gonads, so, it is considered to have a potential effect on treating certain types of infertility [4]. Kisspeptin receptors were primarily defined as GPR54 [5], they belong to G-protein-coupled receptors and resemble the structure of galanin receptor [6]. Binding of kisspeptin to its receptors activates phospholipase C which activates inositol triphosphate and diacylglycerol as secondary intracellular messengers which cause release of intracellular calcium in two phases, rapid and slow phases, and stimulation of protein kinase C [7].Kisspeptin is synthesized in the gonads, pancreas, liver, muscular tissue, kidney, nervous tissue and placenta [8]. kisspeptin receptor-54 is present in the pituitary, placenta, and pancreas [9]. Demirbilek et al [10] and Rhie et al [11] discovered that central and systemic Administration of kisspeptin antagonists inhibited GnRH proofing that kisspeptin neurons act as GnRH pulse generator. In human studies, LH release in women was induced by kisspeptin [12], moreover, in animals, kisspeptins elevated GnRH and LH levels [13]. kisspeptin shows a vital effect in control of the hypothalamic-hypophyseal-gonadal axis and for the onset of puberty and reproduction. Various mutations in gene encoding kisspeptin receptor with inborn hypogonadotropic hypogonadism proposing a basic effect of kisspeptin in puberty, moreover, lately, humans suffering from delayed or lacking puberty attributed to the mutations in gene encoding kisspeptin receptor [14].Irfan et al. [15] discovered that kisspeptin receptors are presented in human and rodent testes and proposed that there is a potentiating effect of kisspeptin on gonadal testosterone secretion stimulated by human chorionic gonadotrophin (hCG). Moreover, Pinto et al. [16] revealed expression of kisspeptin receptors in human spermatozoa and augmented motility and fertilization capacity of sperms on exposure to kisspeptin. They approved that kisspeptin antagonist injection decreased fertilizing abilities of sperms in rats proposing that kisspeptin regulates gonads through peripheral pathway.Kisspeptin derivatives include many types which have a shared binding location to the kisspeptin receptor represented by amino acid length, however, kisspeptin -54 (full-length kisspeptin) is reported to be the derivative with the highest efficacy [17]. Clinical researches about kisspeptin effects is conflicted by the two chief derivatives, kisspeptin -10 and -54, with different pharmacokinetic outlines and routes of administration. The variances between the effects of kisspeptin-10 and -54 are due that kisspeptin-10 has 6-fold shorter plasma half-life in comparison with kisspeptin-54 [18].Many studies previously investigated the acute effect of kisspeptin-54 on the reproductive axis. This research was planned for studying the effect of continuous and prolonged kisspeptin-54 administration on hypothalamic-pituitary gonadal axis (FSH, LH, testosterone), testicular weight, sperm count and motility in adult male rats.
2. Material and Methods
2.1. Experimental Animal
- 20 health adult male albino rats aging 8-10 weeks and weighing 195-240 gram were used. The research methods and experiments followed the rules of ethical committee of Zagazig university. The rats received water and standard chow freely and conditioned in the normal room temperature. During the course of the experiment, body weight, food intake and behavior were assessed daily at the morning between 09, 00 AM and 10, 00 AM [19]. Before starting the experiment and for three days, all animals daily injected subcutaneously with 0.1 ml saline (0.9%) to accommodate to procedures of injection. In day four, animals were classified randomly into two groups (ten rats for each group):Group I (Control Group) in which rats were implanted with Alzet minipumps filled with 0.9% saline. Group II (kisspeptin-54 Treated Group) in which rats were implanted with Alzet minipumps filled with kisspeptin-54 dissolved in 0.9% saline at 4.16 nmol/l.
2.2. Assessment of Viability of Alzet minipumps Preloaded with Kisspeptin-54
- Kisspeptin-54 (Phoenix Pharmaceuticals, INC., USA) stability in preloaded, primed Alzet miniosmoticpumps (Durect corporation, Cupertino, California) over 14 days’ incubation at 37°C was examined by filling the miniosmoticpumps (model-2002) with kisspeptin-54 (4.16 nmol/l), which will be used in this in vivo study [20].
2.3. Methods
- Ten of the preloaded, primed Alzet minipumps were filled with 0.9% saline, other ten pumps were filled with kisspeptin-54 at 4.16 nmol/l under sterile conditions. The used concentration of kisspeptin-54 was dissolved in 0.9% saline to guarantee delivery a rate of 50 nmol/day. At the first day of experiment, all rats were deeply anaesthetized by inhalation of halothane followed with subcutaneous implantation of Alzet minipumps in the interscapular area. In control group, minipumps preloaded with 0.9% saline were inserted in the rats, however, in the second group, minipumps filled with kisspeptin-54 were inserted. The surgical method of implantation of Alzet minipumps was following the procedure of Thompson et al. [17]. A minor cut was done using a sterile knife-edge, spreading the subcutaneous tissue creating a pocket larger than length of the Alzet mninpump, followed with implantation of the pump (flow moderator facing away from the cut). The cut edges of the incision were sutured and closed and the animals were permitted to recover.
2.4. Samples Collection
- Rats were sacrificed by decapitation between 09, 00 AM and 10, 00 AM on day 13 of the study to assure that delivery of kisspeptin-54 sustained for the whole experiment days. Blood of the trunk was sampled, put into heparinized tubes, centrifuged, frozen, and kept at 20°C with avoiding repetitive freezing. removal and examination of the pumps was done to assure complete delivery of the peptide [20]. The testes were dissected and weighed, the epididymis was examined for the sperm count and motility.
2.5. Hormone Assessment
- - Blood follicle stimulating hormone (FSH) level was estimated using ELISA (enzyme linked immunoassay) kits (BioCheck, CA 94404) guide by the technique designated by Rebar et al. (1982). - Blood luteinizing hormone (LH) was estimated using using ELISA (enzyme linked immunoassay) kits (BioCheck, CA 94404) following technique of Tietz (1995).- Blood testosterone was estimated using using ELISA (enzyme linked immunoassay) kits (BioCheck, CA 94404) following technique of Tietz (1995).
2.6. Analysis of Sperm Parameters
- Right epididymis was separated from the animal and crushed in 2 ml of Hank’s buffer salt solution (HBSS) (Sigma-Aldrich Co.-USA) at 37°C [23]. The caudal epididymis sperms were incubated for 5 minutes at 37°C and examined using typical hemocytometer technique. Drawing epididymal fluid into the pipette of White Blood Cells counting till the mark 0.5 followed with drawing of semen diluent (sodium bicarbonate 5 g, formalin 1 ml and distilled water 99.0 ml) till the mark 11 followed by well mixing. A single drop was put in the hemocytometer for one hour and sperms were counted using the light microscope [24]. Total number of sperms, number of live sperms and sperm motility percentage were calculated [25].
2.7. Statistical Analysis
- All analyses were performed using an SPSS program (version 20, SPSS, Inc., Chicago, IL). Results are shown as mean values ± SD. Analysis was performed using one-way analysis of variance (ANOVA). In all cases, P ≤ 0.05 was considered to be statistically significant.
3. Results
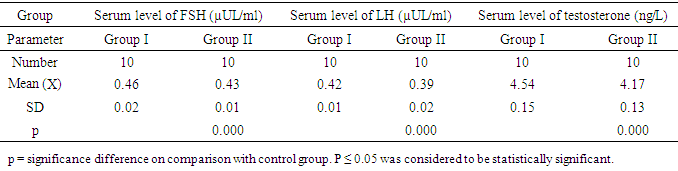
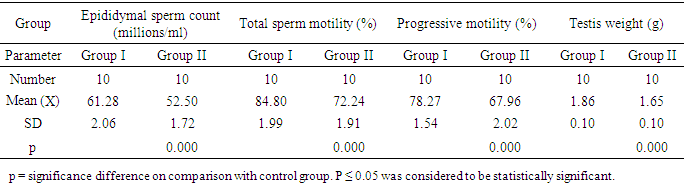
- With daily assessment, it was observed that consumption of food and body weight in the study groups were comparable proposing that the rats were healthy in the whole period of the experiment, moreover, animal behavior showed no adversity along the study period.Table 1 shows Effect of long-term (13-day) S.C. administration of 50 nmol/day kisspeptin-54 in male rats on serum levels of LH, FSH and Testosterone in the studied groups, the results are presented as mean ± standard deviation. In group II (Kisspeptin-54 Treated Group), it was observed that there is significant decrease in mean values (P< 0.001) of the tested hormones on comparing them with group I (Control Group).
|
|
4. Discussion
- Infertility is a great health problematic. Kisspeptin has recently discovered as a controller of the reproductive axis in mammals. Kisspeptin may be used as a new possible therapeutic objective in the control of fertility disorders. Initial studies proposed that exogenous administration of kisspeptin excited release of gonadotrophin in some cases of infertility. Acute intraventricular and peripheral injection of kisspeptin was revealed to excite the hypothalamic-pituitary-gonadal (HPG) axis [4].Many studies investigated the acute effect of administration of kisspeptin on reproduction both centrally and peripherally, however, very little studies investigated the results of prolonged administration of kisspeptin on reproductive axis. This work aimed to concisely explore the results of long term use of kisspeptin as a regulator of reproductive axis and explore effect of kisspeptin on testes and spers viability.In the current study, chronic S.C. injection of 50 nmol/day for 13-days of kisspeptin-54 produced a significant decrease of both plasma LH and FSH when compared to control. The result of this research suggest that prolonged use of kisspeptin may have a desensitizing effect on the hypothalamus and pituitary gland.These findings are consistent with the results of Aytürk et al. [26], who discovered that prolonged kisspeptins administration desensitized the hypothalamic–gonadal–pituitary axis. They reported that plasma LH level was elevated on the second day of injection of Kisspeptin-54 (50 nmol/day), however, its level decreased back to normal level after second day of administration. However, Thompson et al. [17] reported that there was a significant elevation of plasma LH 9 and 12-fold respectively at 60 min following Acute S.C. injection of kisspeptin-54 (1 and 50 nmol) in comparison with the control group. They also stated that plasma LH was elevated following injection of 0.3 nmol kisspeptin-54, however, this elevation was not significant statistically. On examining the results of chronic injection of kisspeptin-54 for 13 days, they found a little change (which is insignificant statistically) on blood levels of LH and FSH on contradiction to the results of this study.The current study revealed that chronic S.C. injection of 50 nmol/day kisspeptin-54 produced a significant decrease of blood level of testosterone. The result of this research suggest that prolonged use of kisspeptin may have a desensitizing effect on the hypothalamus and pituitary gland which subsequently affect gonadal secretion of sex hormones.These results are similar to the findings of Ohga et al. [27] who revealed that testosterone levels were reduced following treatment of adult male rats with kisspeptin-54 for 13-day period. Moreover, Thompson et al. (2006) reported that continuous subcutaneous injection of kisspeptin-54 resulted in a tendency for reduction of total and free testosterone with no statistical significance.However, contradictory results reported by Irfan et al. [15] who investigated that Kisspeptin treatment increased human chorionic gonadotrophin level which stimulated secretion of testosterone in monkeys suggesting that kisspeptin potentiated effect of human chorionic gonadotrophin on gonadal testosterone secretion through peripheral pathway.The current study revealed that chronic S.C. injection of kisspeptin-54 with a daily dose of 50 nmol produced a significantly decrease in the weight of testis, count and motility of epididymal sperm. The result of this research suggest that prolonged use of kisspeptin may have a desensitizing effect on the hypothalamus and pituitary gland which subsequently affect gonadal functions rather than direct effect of kisspeptin on the gonads. This result is parallel to results of Thompson et al. [17] who found a significant decrease in the testicular weight in their experiment of injecting kisspeptin-54 for thirteen days with a daily dose of 50 nmol on comparing with the control group. They claimed that the most outstanding action of prolonged kisspeptin injection was on the testes. They reported that the weight of testis was decreased significantly with a significant degeneration of the seminiferous tubules resulting in damage of both germ and Sertoli cells.However, inconsistent results of Hsu et al. [28] and Pinto et al. [16] who observed that kisspeptin causes biphasic increase in intracellular calcium and motility of human spermatozoa. They proposed that kisspeptin modified fertilizing capacity of mouse spermatozoa through increasing capacitation and that kisspeptin antagonist decreased rates of fertilization of rats’ spermatozoa.
5. Conclusions
- The results of this study showed that prolonged subcutaneous injection of Kisspeptin-54 for thirteen days, with daily dose of 50 nmol significantly reduced plasma levels of FSH, LH, testosterone and testis weight, sperm count and motility which may be attributed to that prolonged administration of Kisspeptin-54 desensitized the hypothalamo-hypophyseal-gonadal axis and consequently led to testicular degeneration in adult rats rather than direct peripheral effect of kisspeptin on the gonads. There is a need to study the direct effect of kisspeptin on the gonads to clarify concisely the usefulness of using kisspeptin as a therapeutic target in treating male infertility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML