-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2018; 7(4): 49-53
doi:10.5923/j.ijim.20180704.01

Safety and Effectiveness of the Use of Luciforte® 500 mg Vial with Patients Admitted to the Intensive Care Unit for Impaired Conscious Level: A Multi-Center, Retrospective, Observational Study
Bassem BG, Sherif M., Mary W. F.
Correspondence to: Mary W. F., .
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Meclofenoxate, the active ingredient of Luciforte® 500 mg, is a cholinergic nootropic used as a drug in the treatment of symptoms of senile dementia and Alzheimer's disease to improve memory and cognitive function. This study was aiming to assess its safety and effectiveness in patients admitted to the intensive care unit (ICU) due to different neurological backgrounds. Methodology: An observational retrospective study was conducted in the ICU of three hospitals. Data of 300 patients who were admitted to the ICU due to central nervous system (CNS) dysfunction and treated with Luciforte® 500 mg Vial twice daily were collected from the ICU records. Results: There was a statistically significant improvement of the level of consciousness as measured by Glasgow coma score (GCS) after treatment; p-value <0.001. The GCS was 11.82 ± 1.60 on ICU admission and 14.57 ± 1.05 on discharge. This improvement was consistent regardless of the cause of disturbed conscious level, the underlying brain lesions, or the baseline APACHE II Score. Patients who showed improvement of GCS were 294 (98.0%) within a median period of 8 (I-Q range = 6-10) days. GCS remained unchanged in only 6 patients (2.0%). No serious or non-serious adverse events were reported within the period of hospital stay. Conclusion: Luciforte® 500 mg vials is a safe, well tolerated and effective drug in improving the conscious level of ICU patients. These results are promising and could justify the conduct of a well-designed randomized controlled clinical trial for assessment of both the short-term as well as the sustained drug effect.
Keywords: Meclofenoxate, ICU patients, Disturbed conscious level
Cite this paper: Bassem BG, Sherif M., Mary W. F., Safety and Effectiveness of the Use of Luciforte® 500 mg Vial with Patients Admitted to the Intensive Care Unit for Impaired Conscious Level: A Multi-Center, Retrospective, Observational Study, International Journal of Internal Medicine, Vol. 7 No. 4, 2018, pp. 49-53. doi: 10.5923/j.ijim.20180704.01.
1. Introduction
- Meclofenoxate, the active ingredient of Luciforte® 500 mg, is a cholinergic nootropic used as a drug in the treatment of symptoms of senile dementia and Alzheimer's disease. [1] It is used specifically to improve memory and to prevent the cognitive deficits associated with dementias and this effect was demonstrated with a double blind clinical trial. [2] It is also used to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Meclofenoxate increases the dimethylethanolamine (DMAE) levels in the brain. DMAE is a precursor of acetylcholine, the important neurotransmitter in the brain that is believed to be responsible for most of the cognitive processes, hence it produces a mental stimulating effect. [3] This drug is generally well tolerated with little of no serious side effects. [4] Meclofenoxate has been found to increase the lifespans of mice by 30–50%, and thus may be used as an anti-aging drug or supplement. [5] Its anti-aging action is due to its effect in significantly reducing lipid oxidation and lipofuscin concentration. [6]The critically ill intensive care unit (ICU) patients, especially those with cerebrovascular stroke and traumatic brain injury, are particularly vulnerable for a significant neurocognitive impairment that will greatly affect their future quality of life. The use of a CNS stimulating agent that may have a protective effect against brain damage may be a plausible option. To the best of our Knowledge, very limited information is available regarding the effectiveness of the use of Luciforte® 500 mg Vial, the brand name of Meclofenoxate available in Egypt, with the ICU patients. This study was aiming to assess the safety and effectiveness of Luciforte® 500 mg Vial in patients admitted to the ICU due to different neurological backgrounds.
2. Subjects and Methods
- Study design and sample:An observational retrospective study was conducted in three hospitals between January-2017 and March-2018. Subjects included were adult patients, ≥18 years and ≤ 75 years, who were admitted to the intensive care unit (ICU) for central nervous system (CNS) dysfunction and treated with Luciforte® 500 mg Vial every twelve hours. Patients with disturbed conscious level due to traumatic brain injury, post-operative delayed recovery, cerebrovascular accident, metabolic causes, and sepsis were included. Patients were excluded from the study if they had a history of hypersensitivity to any of the drug ingredients, their Glasgow Coma Score was 3, were on sedatives during the ICU stay, had severe hemodynamic instability, and who were admitted to the ICU for any organ or system dysfunctions other than CNS. Patients were not treated with any other medication having similar effects. Study data:Data of 300 patients were collected from the ICU records of a one year prior to the study date. Data were retrieved from three centers and included clinical examination with special emphasis on neurological examination (sensory and motor reflexes, Glasgow coma score (GCS)), fundus examination, and central venous pressure, and the radiologic investigations (brain Computed Tomography and Magnetic Resonance Imaging, Electroencephalography, chest x-ray), and laboratory findings (complete blood count and coagulation profile, arterial blood gases and serum electrolytes). Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated for each patient with its equivalent approximate mortality interpretation. Variables definitionsImprovement of patient’s conscious level was considered when the GCS on discharge was more than the GCS on admission.APACHE II Score mortality categories: 0-4: 4% non-operative, 1% post-operative5-9: 8% non-operative, 3% post-operative10-14: 15% non-operative, 7% post-operative15-19: 24% non-operative, 12% post-operative20-24: 40% non-operative, 30% post-operative25-29: 55%: non-operative, 35% post-operative30-34: approximately 73% for both35-100: 85% non-operative, 88% post-operative. [7]Incidence of adverse events was reported with the occurrence of any serious or non-serious adverse event. Research ethics: Approval from the ethical committee review board was taken from the three hospitals before collecting data to be analyzed. Patients’ data were retrieved anonymously for keeping their confidentiality. Statistical analysis:Description of the study sample was performed through calculation of frequency and proportions for categorical variables; and mean and standard deviation, median and interquartile range for quantitative variables. Comparison of the quantitative parameters on admission and on discharge was performed using t-test for paired observations. Comparisons were done among the whole study sample and among the subgroups according to their clinical diagnoses, brain radiological findings, and APACHE II score mortality categories. Chi square test was performed to compare proportions. For all tests, the level of significance was set at P-value ≤0.05. Data management and analysis was performed using IBM Statistical Package for Social Science (SPSS) version 22.
3. Results
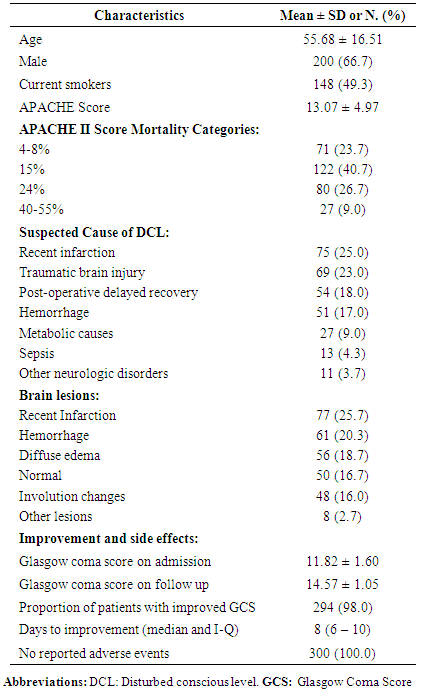
- This study included 300 ICU patients, their mean age was 55.68 ± 16.51 years, male constituted 66.7%, and 49.3% were current smokers. On admission, the mean APACHE II Score was 13.07 ± 4.97, and the “15% APACHE mortality” was the most frequent category (40.7%). Recent infarction and traumatic brain injury constituted around half of the underlying suspected diagnoses for DCL, followed by post-operative delayed recovery (18.0%). The most frequently detected lesions on brain radiology were recent infarction (25.7%), hemorrhage (20.3%), and diffuse edema of the brain (18.7%). GCS was 11.82 ± 1.60 on ICU admission and 14.57 ± 1.05 on discharge. Patients who showed improvement of GCS were 294 (98.0%) within a median period of 8 (I-Q range = 6-10) days. GCS remained unchanged in 6 patients (2.0%). No adverse events (serious or non-serious) were reported within the period of hospital stay (table 1).
|
|
|
4. Discussion
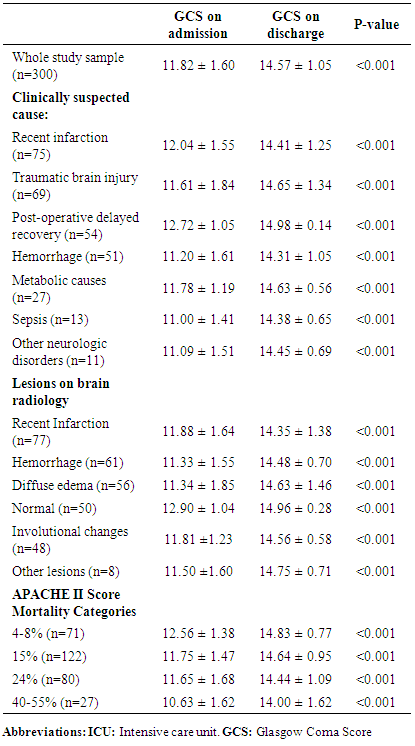
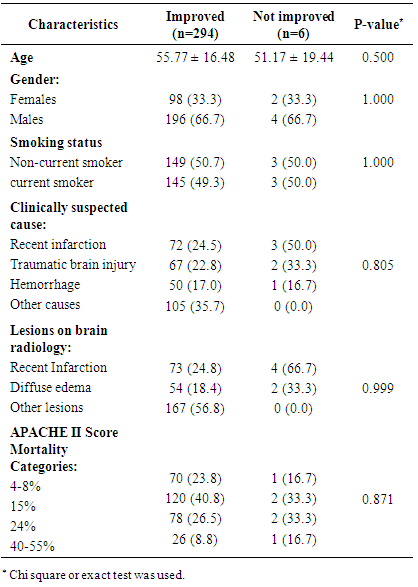
- This study was conducted to evaluate safety and effectiveness of Luciforte® 500 mg vials (Meclofenoxate), not only as an effective nootropic, but also as a neuroprotective agent for ICU admitted patients due to various neurologic background. Significant improvement in the level of consciousness was observed in almost all cases (294/300 patients) treated with the drug in a relatively short period (median=8, I-Q: 6-10 days) with no reported adverse events. The drug effectiveness in improving conscious level was demonstrated when we compare GCS before and after treatment among the whole study sample. To test its effectiveness among various clinically suspected cause of DCL, we reanalyze data comparing GCS before and after treatment among these diagnoses, as separate subgroups of patients; namely, recent cerebral infarction, cerebral hemorrhage, traumatic brain injury, post-operative delayed recovery, metabolic causes, sepsis, and other neurologic disorder. Significant improvement of conscious level was reported with all diagnoses. Reanalysis was also performed among patients’ subgroups according to the radiologically found brain lesions, which were recent Infarction, hemorrhage, diffuse edema, involutional brain changes, normal, and other lesions. Again, significant improvement of conscious level after treatment was observed with all brain lesions groups. The subgroup analysis among patients with the various baseline APACHE II Score mortality categories, 4-8%, 15%, 24%, and 40-55%, revealed the same significant improvement among all categories. Our findings suggested that Luciforte® 500 mg vials was effective in improving the conscious level of ICU patients regardless of their suspected diagnosis, their underlying brain lesions, or their baseline APACHE II Score. Out of 300 treated cases, only 6 patients showed no improvement in their GCS level, which remained unchanged. We tried to explain the reason of this resistance of improvement based on the available data, no significant difference was observed among improved group compared to not improved group regarding any of the demographic or clinical characteristics. Regarding this point, our results were inconclusive, partially because of the very small number of patients who were not improved as well as the limited period of follow up data and its incomplete nature regarding patients’ parameters other than GCS. In fact, Meclofenoxate, the active ingredient of Luciforte®, is one of the original nootropic drugs that are extensively studied along 50 years. It enhances the cognitive and memory function, reduces lipofuscin in the brain thus decreases cell aging, and it improves cerebral blood flow and oxygen in the brain. [3, 8, 9] Studies also demonstrated its effectiveness in treating symptoms of senile dementia and Alzheimer's disease. [1, 4]Meclofenoxate is very safe and high in tolerability. However, possible side effects may rarely include, insomnia, dizziness, restlessness, muscle tremor, depression, nausea, muscle tension, and headache. These side effects may be due to over-dosage and may indicate the need for the dosage to be reduced. [4] This study had many points of strength. First, to the best of our knowledge, it is the first time in Egypt to test the effectiveness of Luciforte® 500 mg as a CNS stimulant for ICU patients. The neurocognitive impairment that frequently encountered with ICU patients may extend beyond their acute phase and hospital stay and lead to significant deficits in their quality of life. This neurocognitive impairments may be understood as a manifestation of occult brain damage secondary to underlying pathophysiological mechanisms related to critical illness. [10] This fact may necessitate considering the ICU patients as brain damaged patients and apply therapeutic tools, such as cognitive stimulation, that have proven effective in treating neurocognitive impairments in acquired brain injury patients. [11] Hence, testing for the effectiveness of a safe CNS stimulant may be justified. The second point of strength is the consistent and rapid effect of the drug that was observed among a relatively large multicenter sample. An important study limitation is the limited time for follow up that included only the period of ICU stay; as this was the period for which we have a complete and accurate records for the patients’ condition. Another limitation is the unavailability of follow up data for parameters other than GCS for better assessment of patients’ condition. In conclusion, Luciforte® 500 mg vials is a safe well tolerated and effective drug in improving the conscious level of ICU patients regardless of their diagnostic category, underlying brain lesion, or APACHE II Score on admission. These results are promising and could justify the conduct of a well-designed randomized controlled clinical trial for assessment of both the short-term effect within the hospital stay as well as the sustained effect after discharge.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML