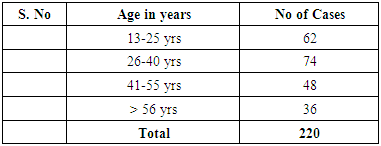G. Rathna Kumar, P. Meena Kumari
Department of Medicine, Tirunelveli Medical College, Tamil Nadu, India
Correspondence to: G. Rathna Kumar, Department of Medicine, Tirunelveli Medical College, Tamil Nadu, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Dengue incidence has increased all over the world. Over 2.5 billion people ie 40% of world population are at risk from dengue.Dengue infection has a wide spectrum of clinical manifestations. Aim: This study aimed to study the age distribution, prevalence pattern of manifestations in Dengue Fever, DHF, DSS and to compare the association of neurological with hepatobiliary manifestations along with prevalence and day of onset of shock. In this prospective observational study, we analyzed 220 dengue patients. The prevalence of hepatic impairment and clinical profile was studied in accordance to dengue fever, dengue hemorrhagic fever and dengue shock syndrome. 79% of cases of dengue infection had hepatic dysfunction. Petechia, jaundice and bleeding were prevalent in the DHF and DSS group. Rise in hematocrit, raised serum alanine transaminase, coagulopathy and low serum globulin indicate severe forms of dengue including DHF and DSS (P<0.001). Gall bladder wall edema was also a predominant finding in dengue infection. 15 patients developed neurological complications; though 13 of them had associated hepatic dysfunction, the results were not statistically significant. Most neurological complications were present during the first three days of illness whereas onset of shock was noted during 4th to 6th days of fever. The study highlights the important clinical, biochemical, hematological and radiological findings which are associated with severe forms of dengue. We conclude that petechiae, jaundice, coagulopathy, low serum globulin levels, increased hematocrit are associated with severe forms of Dengue infection.
Keywords:
DF: Dengue Fever, DHF: Dengue Haemorrhagic Fever, DSS: Dengue Shock Syndrome, AST – Aspartate transaminase, ALT - alanine transaminase, DIC- disseminated intravascular coagulation
Cite this paper: G. Rathna Kumar, P. Meena Kumari, A Study on Prevalence of Encephalopathy in Dengue Patients with Hepatobiliary Manifestations, International Journal of Internal Medicine, Vol. 7 No. 3, 2018, pp. 44-48. doi: 10.5923/j.ijim.20180703.03.
1. Introduction
Dengue fever is an arthropod borne viral disease. It is an important public health problem in India. Dengue fever was first reported from Vellore in Tamil Nadu during 1956. Dengue is widely prevalent in both urban and rural India [1]. Although asymptomatic in most cases, dengue is known to produce plasma leakage, hepatobiliary dysfunction and neurological complications. Dengue is endemic in all states and union territories of India. In 2015, a total of 99,913 dengue cases and 220 deaths were reported from 35 states and union territories of India. Aedes aegypti is the main vector in most urban areas. Aedes albopictus is also a vector in few areas in southern and eastern India. Hepatic dysfunction varies from elevation of transaminase activity to severe hepatocyte injury due to direct effect in liver cells and as a consequence of deranged host immune response [2]. Transaminitis is usually self-limiting. Infection with DENV results usually in undifferentiated viral fever like illness. But in others, it causes dengue fever (DF), Dengue Haemorrhagic Fever (DHF) dengue shock syndrome (DSS) or the expanded dengue syndrome [3]. WHO estimates that there may be 50 -100 million dengue infections worldwide every year. The disease is now endemic in more than 100 countries in Africa, the America, southeast asia, & west pacific countries. Recently, neurological manifestations are reported more frequently. Incidence of neurological complications in dengue fever is 05.- 6% [5]. The neurological complications reported are encephalopathy, encephalitis, Guillain- Barre syndrome (GBS) and myoclonus. [6-8]Though several studies are available on hepatic involvement in dengue infection and neurological complications of dengue fever, not much studies are available regarding the correlation between hepatic and neurological involvement. In this prospective observational study, we describe the hepatobiliary involvement in 220 cases of dengue patients and the prevalence and association of neurological complications in dengue patients with hepatic involvement.
2. Materials & Methods
A total of 220 confirmed cases of dengue were studied. This is a prospective observational study done in fever intensive care unit in Tirunelveli Medical College Hospital, Tamilnadu from July 2017 to December 2017. The dengue patients were classified into dengue fever (DF), dengue haemorrhagic fever (DHF) and Dengue shock syndrome (DSS) according to WHO guidelines. Dengue fever was diagnosed on the basis of positive serum immunoglobulin IgM antibody, analysed by enzyme linked immuno sorbent assay (ELISA).Patients characteristics like age, name, sex, occupation, history, clinical examination were recorded. Laboratory investigations included complete hemogram, sugar, urea, creatinine, liver function tests and coagulation profile. ECG, chest X-ray, Ultrasonogram Abdomen were done for all patients. CT, MRI brain and CSF analysis were done for patients with neurological complications. Patients with known systemic illness like chronic kidney disease, coronary artery disease, hypertension, diabetes, alcoholism were excluded from study.
3. Results
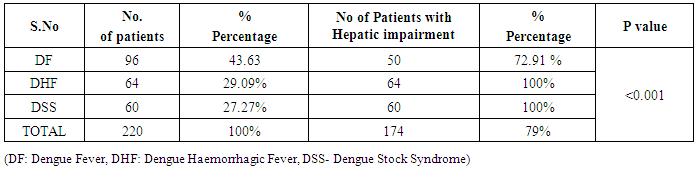
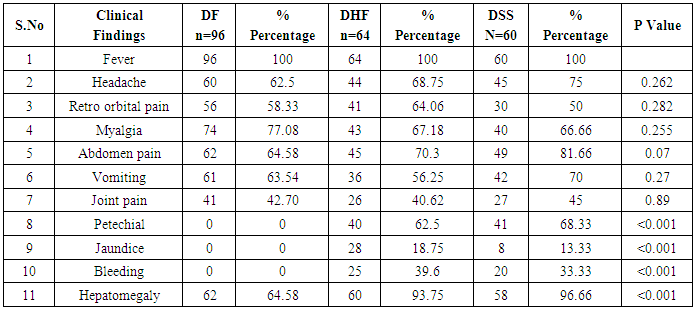
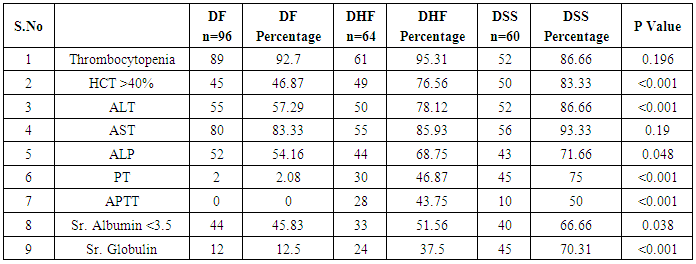
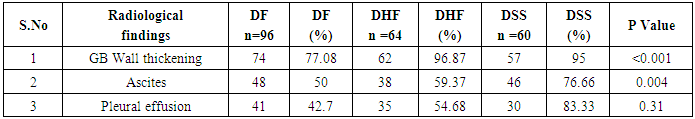
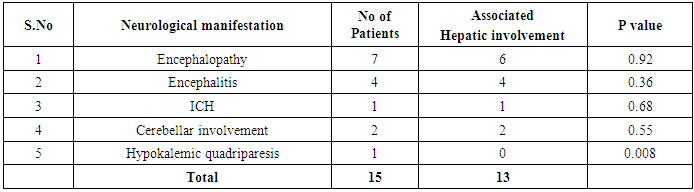
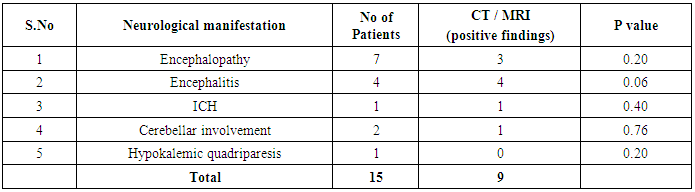
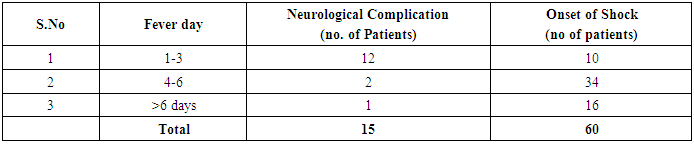
In our study dengue infection was more prevalent in age group of 26 – 40 years followed by 15 -25 years age group (Table 1). 96 patients had dengue fever, 64 patients had dengue hemorrhagic fever and 60 patients were classified as dengue shock syndrome. Hepatic impairment was seen in 50 patients (65.78) with dengue fever (DF). All the patients with DHF & DSS had hepatic impairment. 35% patients with dengue fever did not develop hepatic dysfunction. The results show that hepatic dysfunction is predominant manifestation (Table 2).Analysing the symptomatology and clinical findings, all the patients had fever, 67.7% had headache, 71.3% had myalgia, 70.9% had abdominal pain, 42.72% had joint pain, 57.7% had retro orbital pain and only 18.63% had rash. Hepatomegaly was present in 82% patients (n=180) whereas jaundice was seen only in 16.36% (Table 3).Analysing the haematological profile, 92% cases had thrombocytopenia and 65.45% had haematocrit >40%. 71.36% had raised ALT and 87% had raised AST. Abnormal PT and aPTT were seen in 77 and 38 patients respectively. Coagulopathy was common in patients with DHF and DSS (p<0.001). 53% cases (n=117) had low serum albumin and 36.8% had low serum globulin. Prevalence of low serum albumin and globulin were seen mainly in the DHF & DSS group, in patients who had plasma leakage (Table 4).87.72% (n=193) patients had gall bladder wall thickening (p<0.001) and 65.45% had ascites (n=144) (p=0.004). These associations were found to be statistically significant (Table 5).In our study, out of the 220 patients with dengue infection, 15 patients had neurological manifestations. 13 out of the 15 patients had hepatic dysfunction and 9 of them showed positive radiological findings in CT / MRI Brain. The commonest neurological manifestation in our study was encephalopathy (n=7) followed by dengue encephalitis (n=4), cerebellar infarct (n=2), intracerebral haemorrhage (n=1) and hypokalemic paralysis (n=1). CT / MRI findings included cerebral edema, intracerebral hemorrage, bilateral thalamic infarcts, hypo densities in pons and cerebellum (Table 6), (Table 7). The association between neurological complication and hepatic dysfunction was not statistically significant.We compared the day of occurrence of neurological complication with the day of onset of shock. Neurological complications were common during the first 3 days illness whereas shock was common during 4th to 6th days of illness (Table 8). This study shows that CNS complications precede the onset of shock.Table 1. Distribution of Dengue Patients in relation to age
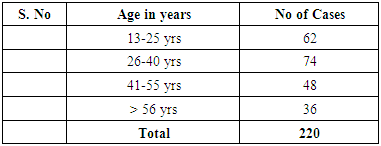 |
| |
|
Table 2. Distribution of dengue patients in relation to hepatic impairment
 |
| |
|
Table 3. Prevalence of clinical features in Dengue patients
 |
| |
|
Table 4. Pattern of haematological & biochemical profile in dengue patients
 |
| |
|
Table 5. Radiological profile in dengue patients
 |
| |
|
Table 6. Prevalence of Neurological manifestations in association with hepatic impairment
 |
| |
|
Table 7. Prevalence and distribution of positive CT/MRI findings in patients with neurological findings
 |
| |
|
Table 8. Prevalence and Comparison of day of occurrence of neurological complication with day of onset of shock
 |
| |
|
4. Discussion
Prevalence of dengue is high in young adults in our study which is in contrast to the study by Bandyopadhyay et al [9]. The above study also showed similar results in the association of hepatic impairment in DF (72.9%) of DHF and DSS (100%). In our study, upto 79% of dengue patient had hepatic impairment. The appearance of jaundice can be due to hepatic injury caused by dengue virus or hypoxia and tissue ischemia due to shock [10]. In our study jaundice occurred in 9.375% which is higher than in the study by Bandyopadhyay.All patients had fever. Symptoms of myalgia, headache, joint pain headache and retroorbital pain were more common in dengue fever group and these results were similar to other studies [11-13].Hepatomegaly is one of the commonest clinical signs of dengue. In the present study 81.8% patients had hepatomegaly. 96.66% of patients with DSS had tender hepatomegaly whereas in a study by Paulo et al, 30% of DSS cases had hepatomegaly [11].In DHF and DSS, acute liver failure occurs rapidly and jaundice can be evident on first day of illness. AST elevation is proportion greater possibly due to monocyte damage. The levels of aminotransferases reach peak at 2nd week [15]. DHF is due to severe liver damage due to direct infection of hepatocytes and Kupffer cells with minimal cytokine response [16]. In our study ALT elevation is seen 73.12% and AST elevation in 81.87%.This result is similar to studies by Mohan et al and Kuo et al [17, 18]. Coagulopathy was more common in DSS followed by DHF in our study. Hepatic cell damage causes decreased production of clotting factors resulting in prolonged PT. APTT is also an important marker of coagulation profile. 10 cases of DSS (16.66%) and 28 cases of DHF (43.75%) had prolonged aPTT. Hypoalbuminemia was commonly noted in DSS (66.66%) followed by DHF (51.56%), though the finding was not statistically significant. Hypoalbuminemia is associated in the severe liver damage in dengue. Low globulin is due to fluid loss into third space which indicates severe damage [10, 19]. Gall bladder wall thickening was seen in 87.72%. In dengue, USG shows gall bladder wall thickening and pericholecystic fluid [20]. Prevalence of neurological manifestations is 6.8% which corresponds to other studies. Sahu et al reported a prevalence of 9.26% [21]. The various tyes of neurological manifestations were comparable to other studies [22-25]. Neurological involvement commonly occurs in their the first three days of infection. Intracerebral hemorrhage is due to direct tissue invasion, capillary haemorrhage, DIC and liver failure. Encephalitis, aseptic meningitis, acute disseminated encephalomyelitis are due to neurovirulent effects of dengue virus. CNS manifestations are due to direct neurotropism, secondary to systemic manifestations, post infectious and immune mediated reaction [5]. Cerebral edema is due to vascular leak, intracerebral hemorrhage, hyponatremia, hepatic encephalopathy and cerebral hypoperfusion secondary to shock [26].
5. Conclusions
We conclude that petechia, jaundice coagulopathy, low serum globulin levels, increased hematocrits are associated with severe forms of dengue. Though dengue patients with neurological complications had associated hepatic dysfunction, the association was not significant.
References
| [1] | Sedhain A, Adhikari S, Regmi S, Chaudhari SK, Shah M, Shrestha B. Fulminant hepatic failure due to dengue. KUMJ. 2011; 9 (34): 73-75. |
| [2] | WHO Comprehensive Guidelines for Prevention and control of Dengue fever and Dengue hemorrhagic fever. SEARO, New Delhi, India: World Health Organisation 2011. |
| [3] | Dengue guidelines for diagnosis, treatment, Prevention and control 2nd ed. General: World Health Organisation 2009. P -144. |
| [4] | Hendarto SK, Hadinegoro SR. Dengue encephalopathy. Paediatr International. Jpn.1992; 4: 350-7. |
| [5] | Verma R, Sharma P et al. Neurological complications of dengue fever: Experience from a tertiary center of north India. Ann Indian Acad Neurol 2011. 14:272-8. |
| [6] | Murthy J. Neurol Comp of dengue infection. Neurol India. 2010; 58:581-4. |
| [7] | Neurological complication of dengue fever in a tertiary care centre. European J of Phermocuticed & Medical Research 2018. 5(6), 408-412. |
| [8] | Fernando S, Wijwickrama A et al. Patterns and Causes of liver involvement in acute dengue infection. BMC Infectious diseases. 2016. 16:319. |
| [9] | Bandyopadhyay D, Chattaraj S et al., A Study on spectrum of hepatobiliary dysfunction and pattern of liver involvement in Dengue infection. J Clinical and Diagnostic research. 2016 May, Vol-10(5): OC21-26. |
| [10] | Seneviratne SL, Malavige GN, de silva HJ. Pathogenesis of liver involvement dengue viral infections. Trans Royal Soc Tropical Med Hyg. 2006; 100: 608-14. |
| [11] | Parkash O, Almas A, Jafri SM et al. Severity of acute hepatitis and its outcome in patients with dengue fever in atertiary care hospital, Karachi, Pakistan. BMC Gastroenterol. 2010; 10:43. |
| [12] | Karoli R, Fatima J, Siddiqi Z et al. Clinical profile of dengue infectuion in a teaching hospital in North India. J infect Dev Countries. 2012; 6:551-4. |
| [13] | Saha AK, Maitra S, Hazra Sch. Spectrum of hepatic dysfunction in 2012 dengue epidemic in Kolkata, West Bengal. Indian J Gastroenterol. 2013; 32:400-3. |
| [14] | Mourao G, Lacerda G, Bastos MS et al. Dengue haemorrhagic fever and acute hepatitis: a case report. Brazilian J of Infectious Diseases. 2004; 8(6). |
| [15] | Shukla V, Chandra A. A study of hepatic dysfunction in dengue. JAPI 213; 61: 460-61. |
| [16] | Macedo C, Nicol AF et al. Histologic, Viral and Molecular correlates of Dengue fever infection of liver using Highly sensitive immunohistochemistry. Diag molecular path. 2006; 15: 223-228. |
| [17] | Mohan B, Patwari AK, Anand VK. Hepatic dysfunction in childhood dengue infection. J Trop Paediatric. 2000; 46: 40-43. |
| [18] | Kuo CH, Tai DI, Chang-Chien CS, Lan CK, Chiou SS, Liaw YF. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992; 47: 265-70. |
| [19] | Villar Centeno LA, Diaz-Quijano FA, Martinez-Vaga RA. Biochemical alterations as markers of dengue hemorragic fever. AMJ Trop Med Hyg 2008; 78(3); 370-4. |
| [20] | Bhatty S, Shaikh NA, Fatima M, Sumbhuani AK. Acute acalculous cholecystis in dengue fever J Pak Med Assoc. 2009; 59(8): 519-21. |
| [21] | Carod-Artal FJ, Wichmann D et al. Neurological complications in dengue viral infection: a prospective cohort study. Neurology. 2014 Oct 28; 83(18): 1601-9. |
| [22] | Ferreira ML, Cavalcanti CG, Coelho CA, Mesquita SD. Neurological Manifestations of Dengue: Study of 41 cases. Arq Neuropsiquiatr. 2005; 63:488-93. |
| [23] | Wasay M, Channa R, Jumani M, Shabbir G, Azeemuddin H, Zafar A. Encephalitis and Myelitis associated with dengue viral infection – clinical and neuroimaging features. Clin Neurol Neurosurg. 2008; 110:635-40. |
| [24] | Misra UK, Kalita J, Syam UK, Dhole TN. Neurological Manifestations of dengue virus infection. J Neurol Sci. 2006; 244: 117-22. |
| [25] | Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B et al. Neurological Manifestations of dengue infection. Lancet. 2000; 25(355): 1053-9. |
| [26] | Varatharaj A. Encephalitis in the clinical spectrum of dengue infection. Neurology India. 2010: 58 (4), 585-591. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML