-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2017; 6(2): 27-32
doi:10.5923/j.ijim.20170602.01

Interleukin-27 is Differentially Associated with CD4+ T Cell Counts in HIV Saudi Patients under Highly Active Antiretroviral Therapy
Haifa M. Al-Nafea1, Nouha M. Hamdy2, 3, Nagwa M. A. Aref4, 5
1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia
2Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3King Saud Medical City, Riyadh, Kingdom of Saudi Arabia
4Department of Botany and Microbiology, College of Science, King Saud University (KSU), Kingdom of Saudi Arabia
5Department of Microbiology, Ain Shams University, Cairo, Egypt
Correspondence to: Nagwa M. A. Aref, Department of Botany and Microbiology, College of Science, King Saud University (KSU), Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The IL-27 as a pleiotropic essential host factor for HIV- infection that modulates both the innate and adaptive immune responses. The study design incorporated sixty-six HIV-1/AIDS Saudi patients under Highly Active Antiretroviral Therapy (HAART1 and HAART2) and other twenty healthy individuals as a control. Flow cytometer estimated lymphocyte immunophenotyping which resembles the total of absolute counts of mature human lymphocyte subsets in whole blood. While plasma IL-27 and other cytokines; INF-γ, IL-2, IL-10, IL-4, IL-6, and TNF-α measured in pg/ml by Flow Immunoassay (Luminex 200). COBAS AmpliPrep assessed the association and quantitation of HIV-1 RNA viral load in plasma. The results indicated considerable significant differences between control and HAART 2 with the element (CD3+8+ T cells) as T-suppressor lymphocyte for the HAART2 with a mean (1109.62), against (779.82) for the control group. There are also considerable differences between control and HAART2 with element (CD3+4+ T cells (as T-helper), CD4+/CD8+ ratio, in favor of scrutiny group with mean (960.41) against (511.17) with element (CD3+4+ T cells), with mean (1.29) against (0.53) with element (CD4+/CD8+ ratio). In our study, viral load in HAART1 and HAART2 decreased below the lower limit of detection with median values of ≤ 20 copies/ml. CD4+ T cells count was indicated 41% change increase in HAART1 while it was 46.8% in HAART2 without any significant changes through one year. The constant dynamic change recorded for antiretroviral (ARV) therapy after 12 months while CD4+ T cells influenced on IL-27 with a percentage increase of 12.00% in HAART2. The results showed enormous differences between control and HAART2 groups with elements (INF-γ, IL-10, IL-2, IL-4, IL-6, IL-27, TNF-α). All of them had strong values at the level of 0.01 while IL-27 were significant at the level of 0.05. The IL-12 family included a heterodimeric cytokine of Interleukin 27 (IL27) which antigen-presenting cells produce it. It plays an important function in regulating the activity of B- and T-lymphocytes. IL-27 had the first protective effect against HIV-1 infection. The present study recommends the potential use plasma cytokines concentrations during acute HIV-1 infection especially IL-27 to predict subsequent disease progression. ARV therapies induce quantitative and qualitative alterations in the cytokine profile of persons that could be warrants attention which causes reductions in AIDS deaths. It is an important Immune-biomedical study for AIDS Saudi patients under medication to be followed up by physicians for managing the AIDS morbidity and mortality in KSA.
Keywords: IL-27, HAART, HIV, CD4+ T cells, KSA
Cite this paper: Haifa M. Al-Nafea, Nouha M. Hamdy, Nagwa M. A. Aref, Interleukin-27 is Differentially Associated with CD4+ T Cell Counts in HIV Saudi Patients under Highly Active Antiretroviral Therapy, International Journal of Internal Medicine, Vol. 6 No. 2, 2017, pp. 27-32. doi: 10.5923/j.ijim.20170602.01.
Article Outline
1. Introduction
- The CD4+ T cells during HIV(human immunodeficiency virus) infection is a risk factor component in the surveillance of disease progression and the response to antiretroviral (ARV) therapy. Therefore, the evolution of HIV/acquired immunodeficiency syndrome (AIDS) global pandemic and the subsequent development of treatment strategies is necessary to prolong the life of infected individuals for developing affordable, reliable and accurate methods for the enumeration of CD4+ T cells [1].The Interleukin (IL)-27 confirmed as an immunomodulatory cytokine with critical roles in both the innate and adaptive immune systems. Adding IL-27 exogenously to primary cell cultures demonstrated a decrease of HIV-1 replication in some cell types including peripheral blood mononuclear cells (PBMCs), CD4+ T cells, macrophages and dendritic cells in the last five years. IL-27 concentration with approximately 100 times the levels was required to prohibit HIV-1 in vitro found in plasma. Non-Human Primate Models of HIV-1 infection required for testing IL-27 before any human trials as appropriate parameters such as the optimal dose and route of IL-27, kinetics of viral replication following administration and possible adverse effects. For preclinical studies would be the next step for evaluation the effectiveness and potency of IL-27 as a therapeutic agent [2].Significant elevation in plasma IL-27 confirmed for HIV-positive individuals. IL-27 was positively linked with CD4+ T cells and negatively correlated with HIV viral load in HIV-mono-infected patients. Moreover, it altered the correlations between HIV viral loads, IL-27 titers, and CD4+ T cell counts. IL-27 varies in treatment-naive groups that had HIV mono-infections. It is critical information to be taken into consideration when caring and treating those with HIV mono-infection and HIV/ hepatitis C virus (HCV) co-infection [3].The plasma levels of cytokines or chemokines associated with immune activation might also be biomarkers of an increased risk of mortality likelihood of death in HIV-infected individuals. They concluded that the higher plasma levels of proinflammatory cytokines (IL6 and Tumor necrosis factor-alpha (TNF-α)) were related to an increased risk of all-cause mortality [4]. Some cytokines secreted during HIV infection that enhances both innate and adaptive immune responses. Interferons (IFN)-α/β/γ, IL-12, IL-15, and IL-18 have been found to contribute to the development, maturation, differentiation, and function of natural killer (NK) and other immune cells. Nuvor et al., 2016 found the concentration of IFN-γ were equal at the CD4+ T cell division except for an increase in HIV-2 infected patients (90 HIV-2 infected) at low CD4+ T cell counts (P= 0.02). The results were similar in all HIV-1 subjects. So there is variability in the plasma levels of innate cytokines at different grades of HIV infection. However, the result of elevated IFN-α in HIV-2 infected asymptomatic citizen is harmonious with the high innate NK responses previously noted at this grade of infection [5].Recent studies from samples enrolled in 252 individuals indicated that the levels of pro-inflammatory cytokines not normalized in the blood instead of being HIV RNA suppression with less than 50 copies/ml during ARV therapy. It was through the measurement of 31 cytokine/chemokine concentrations by multiplex bead array in blood and seminal plasma collected. [6].CD4+ T cells during HIV infection is a risk factor component in the monitoring of disease progression and the response to antiretroviral (ARV) therapy. Therefore, the evolution of HIV/AIDS global pandemic and the subsequent development of treatment strategies is necessary to prolong the life of infected individuals for developing affordable, reliable and accurate methods for the enumeration of CD4+ T cells [1]. CD4+ T cell population with well documented current regimens increased significantly and led to increased the life expectancy of infected individuals [7].Most highly active anti-retroviral therapy (HAART) models are extremely sensitive to small changes in drug efficacy. The difficulties with drug penetrance and maintenance of effective intracellular drug concentrations are risk factors in all cells susceptible to HIV infection. It may underlie ongoing viral replication; the immune system might have a role in the persistence of HIV replication. That could guide a clinician’s decision to change the drugs used in HAART for a particular patient [8].The present study aims to explore the importance of IL-27 as a pleiotropic essential host factor required to inhibit HIV-1 in plasma for HIV- infection that modulates both the innate and adaptive immune responses. The evaluation of integrated association with other cytokines levels was analyzed. As IL-27 also targets macrophages, it may prove to be useful for attacking HIV-1 in this reservoir.
2. Materials and Methods
2.1. Study Participants
- The study design consisted of a case-controlled study. The total of sixty-six AIDS patients was under HAART and twenty healthy individuals as a control. All subjects were Saudi from the KSA. All control who screened as HIV negative and HCV negative. HIV-1/AIDS patients with an average age 20-50 years were chosen from HIV clinic according to RT-PCR test in King Saud Medical City in Riyadh. All the AIDS patients diagnosed according to the “AIDS Diagnose and Treatment Guidelines.” Also, all patients were undertreated with HAART for six months and considered as an HAART1 group then patients were scheduled to visit the clinic routinely for another half as an HAART2 group.
2.2. Quantification of HIV Viral Loads
- Whole blood specimens collected from all subjects including patients and controls into EDTA containing tubes. Plasma stored at -80°C until analysis. The COBAS AmpliPrep/COBAS TaqMan HIV-1 Test is an in vitro nucleic acid amplification assessment for the quantitation of HIV-1 RNA in human plasma using the COBAS AmpliPrep instrument for automatic specimen processing and the COBAS TaqMan 96 analyzer for electronic amplification and detection. The test can quantitate HIV-1 RNA over the range of 20-10,000,000 copies/mL (33 to1.67x107 International Units/l=mL).
2.3. CD4+ Counting
- Four-color fluorescence kit assessed CD4+ T cell count of HIV-positive individuals and controls for CD4/CD8/CD3 (BD Multitest TM IMK kit) counting according to the manufacturer’s instructions. Fresh whole blood was analyzed on BD FACS Canto II flow cytometer within eight hours. Multiset software performed analysis of CD4/CD8/CD3. The results of immunophenotype were reported as the percentage(%) of positive cells per lymphocyte population or as the number (absolute count) of active cells per microliter of blood (cells/µL). Because we used BD FACS Canto II clinical software, the total counts were determined automatically by software (We use the BD FACS Canto IITM flow cytometer).
2.4. Plasma IL-27 Level
- IL-27 titer and other cytokines; IL INF-γ, IL-2, IL-10, IL-4, IL-6, and TNF-α were measured in plasma with EMD Millipore’s MILLIPLEX MAP Human Th17 Magnetic Bead Kit according to manufacturer’s instructions. The concentration of plasma cytokines in pg/ml.
2.5. Statistical Analysis
- Results for different variables summarized as a mean and standard deviation. Data were analyzed using the software Statistical Package for Social Sciences (SPSS) version 17.0, USA. Independent Sample T-Test utilized for the comparison of plasma IL-27 and other cytokines between HIV-infected samples and healthy controls. A P value lower than 0.15 reflects a trend of clinical relevance and a p-value of less than 0.05 was specified as statistically significant [9]. Multiple-Regression used for the effect of some variable (age, sex) on some interesting variable.
3. Results
3.1. Age and Gender of Participants
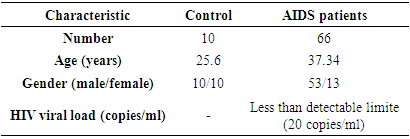
- The age and sex of the patients enrolled in the study outlined in Table 1. The mean age was 25.6 years (20-39 years) for the control group, whereas, the average age of HIV-1AIDS patients was 37.34 years (median 37 and range 24.0-50.0). The control group consists of an equal number of males and females (n=10 each), whereas, most (80%, n=53) patients were male in the HIV-1/AIDS treatment group. There was a statistically significant gender disparity between the two cohorts with a male preponderance in the treatment group (P=0.003).
|
3.2. IL-27 Levels in HIV-infected Persons
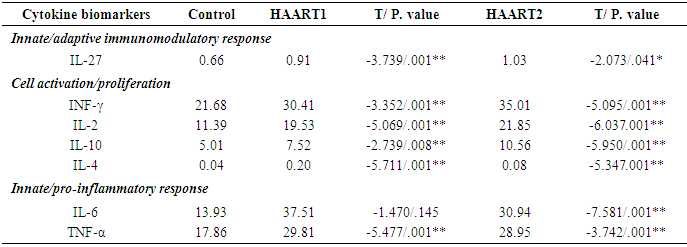
- After that plate run on Luminex 200 with an xPONENT software program. Save and analyze the Median Fluorescent Intensity (MFI) data using a 5-parameter logistic or spline curve-fitting method for calculating analyte concentrations in samples. The results were obtained with Luminex with an xPONENT software program (xPONENT 3.1) and analyzed with the MILLIPLEX Analyst (EMD Millipore) using a standard curve derived from recombinant cytokine. IL-27 levels were among 0.66pg/ml in the control group and 0.91 and 1.03 pg/ml in AIDS patients. Compared to healthy controls, the IL-27 level was significantly higher in HAART1 individuals (P<0.01) and HAART2 (P<.041). While all other studies cytokines were significant in HAART1 except IL-6. on other side HAART2 revealed significant data for all of them (Table 2).
|
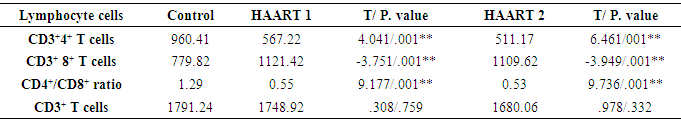
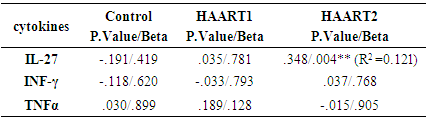
3.3. Correlation of Plasma IL-27 Titers and CD4+ T Cell Counts
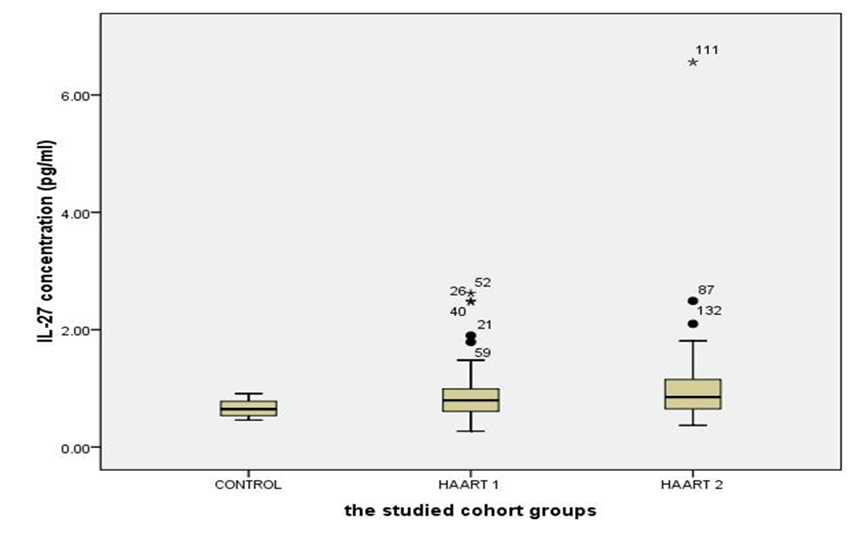
- The number of CD3+4+ T cells and CD4+/CD8+ ratio were highly significantly lower than controls after HARRT1 treatment (P≤ 0.001). The same situation was for HAART2 (P≤ 0.001) Table 3. It was an effect of CD3+4+ T cells on the level of IL-27 in HAART2 group since the indication level value attained (0.004 in Figure 1). Also, the results showed that the correlation coefficient 's value was (0.348), and this indicated that there is a significant direct relation between the two variables. Also, the results showed that the value (R2) equals 0.121, this means that the CD3+4+ T cells Influences on a variable (IL-27) with a percentage of (12.00%), and the remaining percentage dues to the other factors (Table 4). Control group attained 0.66 Pg/ml for IL-27. Since the average of HAART1 group attained (0.91) with a difference (0.25) and variation rate (37.88%), since the mean of HAART2 group reached (1.03) with a difference (0.37), and the variation rate of (56.0%) against a control group. In addition to, the increase in higher values of IL-27 in the Box Plot with maximum high level (6.56) compared to control (Figure 2).
|
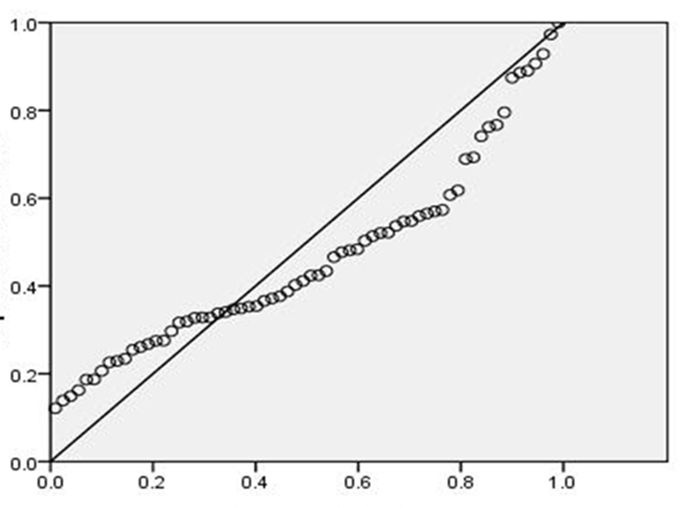 | Figure 1. Effect of CD3+4+ T cells on the IL-27 in an HAART2 group with at level P= 0.004 |
|
4. Discussion
- [10] Moreover, [11] proved that HIV-1 caused deleterious effects to the host mainly through the progressive loss of CD4+ T cells eventually leading to the development of AIDS. Evidence of residual inflammation or increased immune activation exists, even in patients with HIV with adequate CD4+ T-cell restoration on antiretroviral therapy. Markers of residual inflammation in patients with HIV on antiretroviral therapy significantly associated with mortality [12].In the present study (Table 3) showed the same data concerning the count of CD4+T cells. The mean was 567.22 cells/µl with high significantly lower than controls which were 960.41 cells/µl after HARRT1 treatment (P≤ 0.01). CD4+ T cell for HAART2 was 511.17cells/µl. [10] The study supported our results for HAART treatment. Total CD4+ T cells were elevated significantly at 6 and 12 months after HAART treatment in AIDS patients. However, were still lower compared to control (CD4+ T cells in control 775±150, in AIDS patients after HAART for six months CD4+ T cells was 289± 137, and after 12 months of HAART CD4+ T cells was 384 ±384.The optimum patients initiating HAART with CD4+ T cells counts was found to be 350–500 cells/mm3 that had a 94% increased risk of death by [13] in a large cohort study, about those with baseline CD4 counts above 500 cells/mm3. Another study documented an increased risk of death when HAART deferred until the CD4+ T cells count fell below 350 cells/mm3 [13, 14] this was the same initiation HAART responses to antiretroviral therapy in our study to be followed as previously mentioned. In our study, we followed the same guideline for [15] who stated during the diagnosis of HIV infection in KSA for the first time based on international HIV treatment guidelines at least recommended the use of ART in the patients whose CD4+ T cells count be ≤ 350, especially since 2011. With more active ART therapy. That was a critical attitude goes with [16] who proved that using correct ART results in rapid control of HIV and partial restoration of immune function, leading to prevention of the various complications that define AIDS.Interleukin 27 (IL27) is a heterodimeric cytokine related to the IL-12 family that IL-27 produced by antigen-presenting cells. It plays an important function in regulating the activity of B- and T-lymphocytes. The first indication that IL-27 had a protective effect against HIV-1 infection was observed in a model system using noninfectious human papilloma virus-like particles (VLPs) as a cervical cancer vaccine [17]. The IL-27 may inhibit HIV-1 replication within T cells following the transfer of HIV-1 from infected dendritic cells [2]. However, a recent study has revealed a novel IL-27 signaling pathway in macrophages and also characterized an essential host factor which is critical for HIV-1 replication [18]. Few studies are investigating the correlation between systemic levels of IL-27 and correlate of immune dysfunction seen with HIV-1 infection. One study examined the plasma levels of IL- 27 in patients with HIV-1 infection alone or co-infected with HIV-1 and hepatitis C [9].There was a slight negative correlation between IL-27 plasma levels and HIV-1 viral load. [2]. the present study indicated HIV-1 low viral load level (median was less than 20 copies/ml) in HAART1 and HAART1reflected the same previous data of IL-27 that had a high significant increase in HAART1 (P<0.01) more than HAART2 (P<0.05) in its level in both of them. The effects of IL-27 interpreted its eliciting by its interaction with a particular cell-surface receptor complex possessed of two proteins known as IL27R and gp130. The IL-27 is a promising therapeutic agent in HIV-1 infection because the anti-HIV effects demonstrated in multiple cells targeted by HIV-1 including CD4+ T cells, macrophages, and other cells. These in vitro studies which suggested that the effective concentration required suppressing HIV-1 replication is in order of 50–100 ng/ml. It is approximately 100–1000 times the levels of IL-27 found in plasma. The key question is whether these concentrations achieved its effect in vitro and extended in vivo [2]. In our case study for AIDS patients who treated with HAART, CD3+4+ T cells influenced on IL-27 with a percentage of 12.00% in HAART2. A reasonable direct relation at level 0.004 with a mean level of potent cytokine IL-27 (1.03 pg/ml) was lower than 1000 times the value mentioned by Swaminathan S et al. with the association to CD3+4+ T cell count (511.17 cells/µl).
ACKNOWLEDGEMENTS
- Special acknowledgment to Research Center of Female Scientific and Medical Colleges, Deanship of Scientific Research, KSU due to this research project supported by a grant by them.
Abbreviations
- HIV, human immunodeficiency virus; ARV, Antiretroviral; AIDS, acquired immunodeficiency syndrome; Interleukin, IL; TNF-α, Tumour Necrosis Factor alpha; IFN-γ, interferon gamma; NK lymphocyte; NK, natural killer; HAART, highly active antiretroviral therapy; CD3+, mature human T lymphocytes; CD3+CD4+, helper/ inducer T lymphocytes; CD3+ CD8+, suppressor/cytotoxic T lymphocytes; PBMCs, peripheral blood mononuclear cell.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML