-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2017; 6(1): 16-26
doi:10.5923/j.ijim.20170601.03

Prevalence and Determinants of Left Ventricular Geometric and Functional Abnormalities in Asymptomatic Hypertensive Adults at a Tertiary Hospital, South-Southern Nigeria
Unamba Ndubuisi Norbert1, Akpa Maclean Romokere2, Odia Osaretin J.3
1Department of Medicine, University of Portharcourt Teaching Hospital, Portharcourt, Nigeria
2Department of Medicine, Faculty of Clinical Sciences, University of Portharcourt, Portharcourt, Nigeria
3Department of Internal Medicine, Faculty of Clinical Sciences, University of Portharcourt, Portharcourt, Nigeria
Correspondence to: Unamba Ndubuisi Norbert, Department of Medicine, University of Portharcourt Teaching Hospital, Portharcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

BACKGROUND: Hypertensive left ventricular hypertrophy (LVH) is a predictor of cardiovascular morbidity and mortality. Left ventricular geometric pattern further increases the cardiovascular disease burden among hypertensives. The aim of this study was to determine the prevalence, determinants, pattern of left ventricular geometry, and left ventricular (LV) functional status of hypertensives, pre-hypertensive, and normotensive Nigerians. METHOD: A cross-sectional comparative study conducted at the University of Port Harcourt Teaching Hospital, Rivers state, Nigeria. Clinical and trans-thoracic echocardiography was performed among recruited patients with normal blood pressure, pre-hypertension, and newly-diagnosed hypertension. Two socioeconomic indicators (education and occupation) were employed to estimate the effect of socioeconomic status (SES) on LV structure and geometry. RESULTS: A total of 218 subjects were studied, 121 newly-diagnosed hypertensives, 60 pre-hypertensives, and 37 normotensives. The mean age of the hypertensive patients was 51.32±13.1 years, the pre-hypertensives was 50.25±13.0 years, while the normotensives was 47.76±12.0 years. This study showed that individuals within the lower SES had higher blood pressure (BP) levels. Lower educational class was inversely associated with the presence of LVH and LV remodeling. This study also found that central obesity was more prevalent among the lower social classes than among the upper and middle class (p=0.020). Subjects with pre-hypertension had higher values of most echocardiographic parameters than those with normotension. The prevalence of concentric remodeling, eccentric hypertrophy and concentric hypertrophy were 28.8%, 5.85, and 34.6% in the pre-hypertension group, and 16.9%, 14.6%, and 65.2% in the hypertension group. Logistic regression analysis showed that only diastolic blood pressure (DBP) was an independent risk factor for concentric remodeling, concentric hypertrophy, and eccentric hypertrophy (OR=0.257, p=0.006). This study also showed that impaired LV diastolic dysfunction occurred earlier than systolic dysfunction with an associated greater atrial contribution to LV filling. CONCLUSION: In countries undergoing epidemiological transition, effects of socioeconomic status on blood pressure and other cardiovascular risk factor may require more than a casual assessment. Left ventricular geometrical changes exist in adults with pre-hypertension and hypertension in South-Southern Nigeria and may be influenced by social stratification. The geometric changes are strongest among the lower social classes and may be due to higher BP levels. There is a need to develop and test appropriate social interventions to correct social inequalities among the populations to reduce the impact of social factors on cardiovascular health.
Keywords: Left ventricular hypertrophy, Geometric pattern, and functional abnormalities
Cite this paper: Unamba Ndubuisi Norbert, Akpa Maclean Romokere, Odia Osaretin J., Prevalence and Determinants of Left Ventricular Geometric and Functional Abnormalities in Asymptomatic Hypertensive Adults at a Tertiary Hospital, South-Southern Nigeria, International Journal of Internal Medicine, Vol. 6 No. 1, 2017, pp. 16-26. doi: 10.5923/j.ijim.20170601.03.
1. Background
- Hypertension is one of the primary risk factors for cardiovascular disease [1]. It is currently the commonest cardiovascular disease in black Africans [2, 3]. Structural changes occur in the heart and vasculature as a consequence of established hypertension [4]. In hypertensive patients, an adaptive myocardial response to increased cardiac afterload results in left ventricular hypertrophy (LVH) [5].Hypertensive LVH is a powerful independent predictor for sudden cardiac death [6], ventricular arrhythmias [7], myocardial ischemia [8], coronary heart disease [9], heart failure [10], as well as ischemic stroke [11]. LVH is defined as an increase in the mass of the left ventricle, which can be secondary to an increase in wall thickness, an increase in cavity size, or both [12]. In addition to the absolute increase in left ventricular mass (LVM), the pattern of structural adaptation of the left ventricle is influenced by the type of hemodynamic load to which it is subjected to. Therefore, of even greater prognostic importance are the various types of geometric patterns that may arise as a consequence of the hypertension [13]. Classification of the left ventricular geometric pattern using the left ventricular mass index (LVMI) and the relative wall thickness (RWT) yields the following patterns: normal, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy [14]. It has been found that concentric hypertrophy appears to carry the highest risk and eccentric hypertrophy an intermediate risk [15]. Systolic and diastolic function of the left ventricle can also be significantly influenced by the geometrical patterns which are also important in cardiovascular prognosis [16].Although these subjects do not have symptoms as a prerequisite for recruitment, some geometrical and functional changes of the heart may be detected by echocardiography. Some investigators had reported that untreated hypertensive patients had higher prevalence of geometrical and functional changes in left heart [17, 18], but data regarding the association between overt hypertensive/ pre-hypertensive patients and geometrical and functional changes of left heart are rare, especially in adults in South-Southern Nigeria.
2. Methods
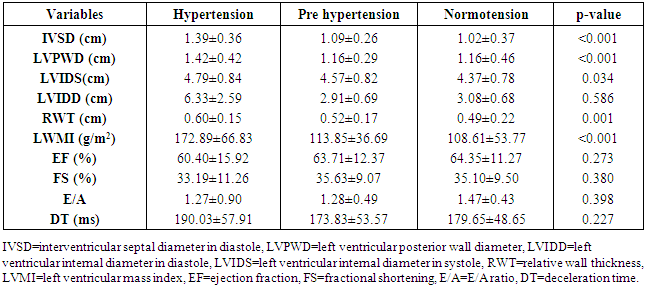
- Study populationStudy subjects were randomly recruited from newly diagnosed and treatment naïve hypertensive patients attending the general out-patients, and medical out-patients clinics of the University of Port-Harcourt Teaching Hospital from January 2016 to August 2016. Those who were currently taking lipid-lowering drugs, contraceptive pills, and diabetics were excluded from the study. All participants underwent a routine clinical examination, blood biochemical examination and trans-thoracic echocardiography. Finally, 218 subjects were recruited as cases. These were further stratified into two groups of 121 persons with newly diagnosed hypertensive patients, 60 pre-hypertensive patients, and 37 normotensive individuals. Hypertension was defined as diastolic blood pressure (DBP) of ≥90 mmHg, and/or systolic blood pressure (SBP) of ≥140 mmHg, pre-hypertension as SBP of 120–139 mmHg and (or) DBP of 80–89 mmHg [19] as defined by JNC7. Written informed consent was obtained from participants and the ethical committee of the hospital.Demographic and clinical characteristicsDemographic and clinical characteristics such as age, gender, duration of hypertension and current anti-hypertension therapy were obtained by a structured questionnaire. Blood pressure was measured with a standard mercury sphygmomanometer according to standard protocol [20]. Height, weight, waist circumference, hip circumference were measured manually. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Waist-to-hip ratio was also calculated.Socioeconomic status variables:Education and occupation were used as parameters for socioeconomic status (SES). Education was stratified into 4 groups: none, primary level, secondary level, or tertiary level. Occupation was stratified into six groups: upper class, middle class, junior management, skilled manual occupation, semi-skilled class, and the unskilled class.Laboratory examinationFasting venous blood were collected and analyzed in the chemical pathology laboratory of the University of Port Harcourt Teaching Hospital for lipid profile and blood glucose. Fasting cholesterol and triglyceride levels were measured using the enzymatic method. Fasting HDL-C was measured with the precipitation method. LDL-C values were calculated using the Friedewald equation when triglyceride level was less than 4.0mmol/L: LDL-C= TCH- (HDL-C+TG/2.2) [21].A trans-thoracic echocardiographic study was done for all the patients with an Aloka Prosound SSD 4000 echocardiography machine equipped with a 2.5MHz transducer. With the patient in the left lateral decubitus position, targeted echocardiographic estimations were taken. These included the standard 2-Dimensional Oriented Motion-mode measurements of interventricular septal thickness in diastole, left ventricular posterior wall thickness in diastole, and left ventricular end diastolic diameter just beyond the tips of the mitral valve leaflets. Left ventricular mass was calculated using the American society of Echocardiography formula modified by Devereux [22].LVM (gm) = 0.8 x [1.04 (LVIDd + PWTd+ IVSd)3– LVIDd3] + 0.6gWhere:LVIDd = Left ventricle internal diameter in diastole PWTd = Posterior wall thickness in diastole, andIVSd = Interventricular septal thickness in diastole. Left ventricular mass was indexed to body surface area using cut-off values of 134g/m2 and 110g/m2 for men and women respectively. [22]Relative wall thickness (RWT= 2 × posterior wall thickness in end diastole/LV diastolic diameter in end diastole) was calculated. A partition value of 0.45 for RWT was used for both men and women [23]. Patients with increased LV mass index and increased RWT were considered to have concentric hypertrophy, and those with increased LV mass index and normal RWT were considered to have eccentric hypertrophy. Those with normal LV mass index and increased or normal RWT were considered to have concentric remodeling or normal geometry, respectively.Statistical analysisData was expressed as mean± standard deviations and frequencies as a percentage. Continuous variables were compared with the Students t-test or one-way analysis of variance (ANOVA) as considered appropriate. Proportions or categorical variables were compared with the Chi-square test. Relations among continuous variables were assessed using Pearson correlation coefficient and linear regression analysis. Multiple logistic models were constructed to elucidate the independent determinants of LV remodeling. The odds ratio and 95% confidence intervals were calculated. All analyses were performed by SPSS statistical software (version 19.0, SPSS Inc). P values of <0.05 were considered statistically significant.
3. Results
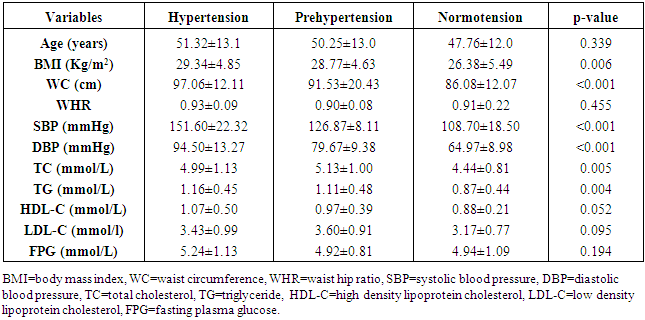
- Baseline clinical characteristics and biochemical parameters of total subjects are shown in Table 1. The age of the study participants ranged between 20 and 86 years with a mean age of the hypertensive patients being 51.32±13.1 years, the pre-hypertensive cohorts 50.25±13.0 years, and the normotensives 47.76±12.0 years (p=0.339). Majority [66.5%] of the participants were in the 40-59 years’ age-group.
|
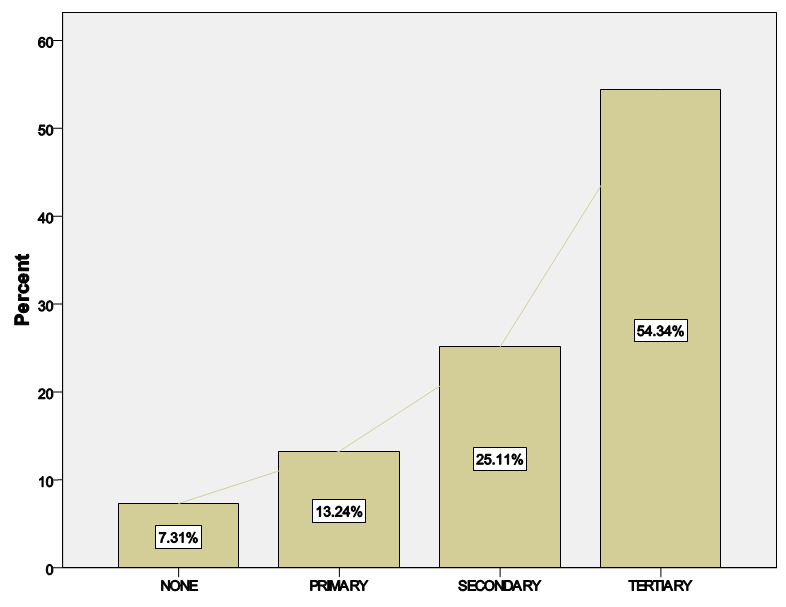 | Figure 1. Distribution of educational status across the population |
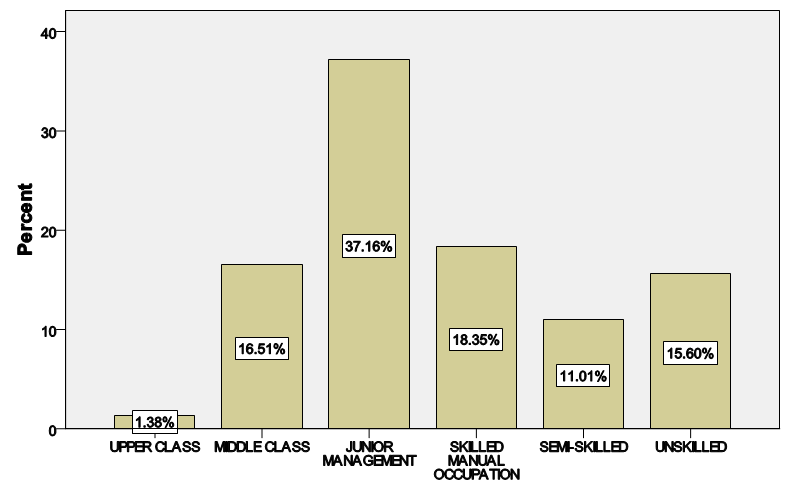 | Figure 2. Distribution of the study population according to social class |
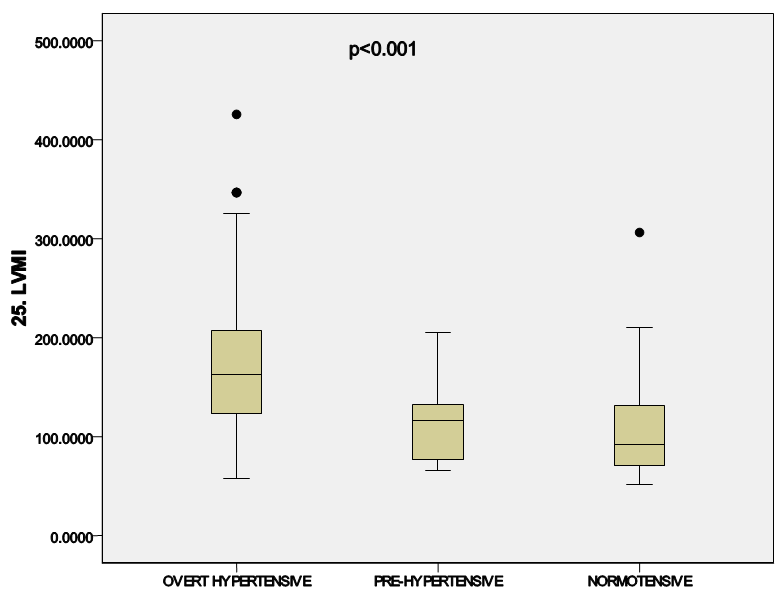 | Figure 3. Diagram showing the mean LVMI across the groups |
|
|
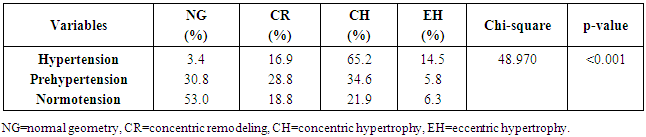
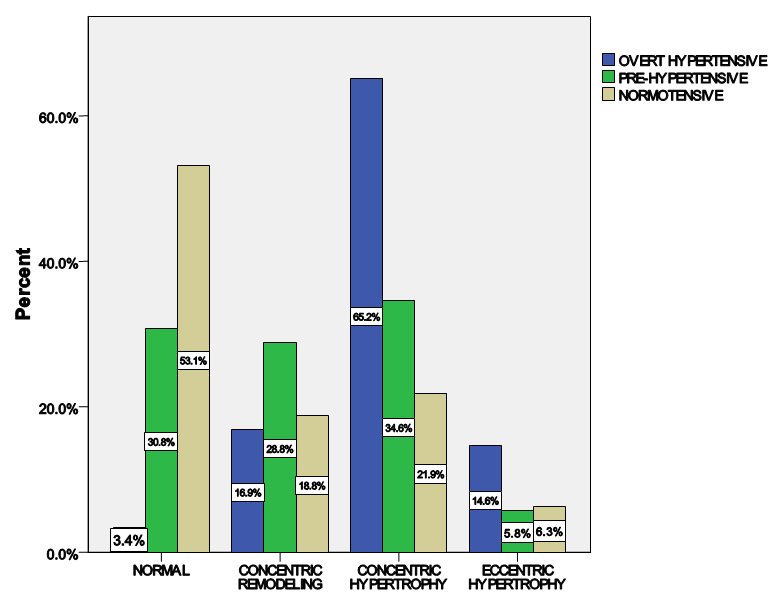 | Figure 4. Distribution of LV geometric patterns across the groups |
|
|
4. Discussion
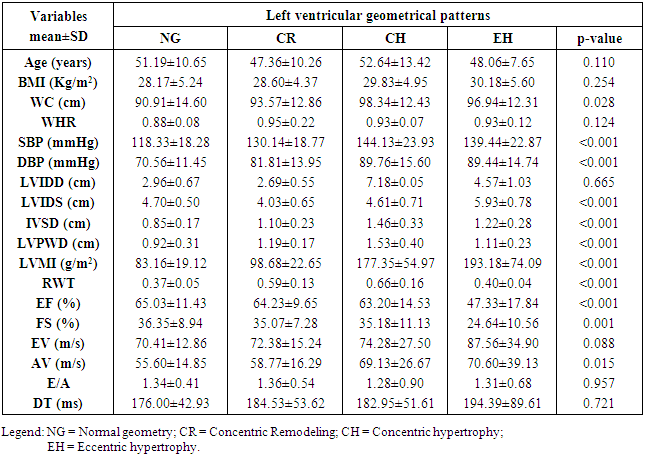
- LVH was detected in 80.9% of subjects with hypertension but down to 43.4% of pre-hypertensives, and lowest among the normotensive subjects 29%. This finding is similar to what was reported by Stabouli et al. who reported a higher prevalence of LVH among pre-hypertensive and hypertensive children than normotensives [24]. The higher values reported in this study when compared to that reported by Saidu et al [25] despite a similar trend may be due to differences in intrinsic population characteristics of a given geographical location.In this study, abnormal LV geometry was found in 79.2% of the entire study population. This was figure was slightly higher than 70% reported by Silangei et al [26] among treated and untreated hypertensives, 72% reported by Aje et al [27] and 75% reported by Isa et al [28] in newly diagnosed hypertensives.The patterns of LV geometry in the entire study population were as follows: normal geometry (20.8%), concentric remodeling (20.8%), concentric hypertrophy (48.0%), and eccentric hypertrophy (10.4%).LV remodeling was found in all the groups with concentric and eccentric hypertrophy predominating in the hypertensive group (65.2% vs. 14.8%), while concentric hypertrophy was more prevalent in the pre-hypertensive group (34.6%). Majority of the hypertensives in our study had concentric LV hypertrophy which was similar to the findings by other African investigators [26-28]. Other studies especially among Caucasians and Asians hypertensives reported eccentric hypertrophy as the prevalent LV geometric pattern [29, 30]. Although traditional cardiovascular risk factors form the basis of most cardiovascular risk predictions, these factors have been reported to explain only a fraction of the overall risk for cardiovascular disease (CVD) [31]. Across the globe socio-economic inequalities have been implicated as a cause of CVD [32]. A gradient of incidence of CVD mortality and morbidity and mortality has been reported across the spectrum of SES. This is mainly defined by income, occupation and educational status [33]. W e found that most (37.16%) of our study populations were in the lower social class (Figure 2). This study also revealed that most of the individuals with a higher level of education were of the higher socioeconomic class. This is important because it is a fact that socioeconomically disadvantaged groups are well associated with coronary heart disease and CVD mortality [34]. It has been reported that SES is closely related with quality of diet [35]. Low socioeconomic groups are known to prefer white bread, potatoes and rice [36] compared to those of high SES, who prefer wheat or wholegrain products that have low glycemic index as well as a greater amount of fibre [37]. The food types eaten by the low socioeconomic groups because of their high glycemic index lead to the accumulation of advanced glycosylated end-products (AGES) which have implicated in LVH [38]. This might partly explain why the subjects in the lower socioeconomic class in this study had a higher prevalence of LVH.In addition, we found that the socioeconomic class also had an influence on LV geometry, with 91.7% of those with no formal education have some form of LV remodeling. This might be explained by the fact that people with higher SES tend to follow a rather more modern dietary pattern, while those belonging to the lower SES tend to adopt the more traditional dietary pattern [39]. Hiza et.al using the Health Eating Index-2005 as a means of measuring dietary quality demonstrated that adults with a tertiary level of education had higher scores for the consumption of whole fruit, total vegetables, whole grains, and calories from solid fats, alcoholic beverages compared to all education levels. Those with less than secondary school education had a lower score for oils and higher scores for saturated fat and sodium compared with all other education levels [40]. These individuals in the lower socioeconomic class are therefore more prone to the risk factors for LVH such as hypertension and central obesity. It is therefore not surprising that the individuals in this study in the lower socioeconomic groups had a higher prevalence of central obesity and mean SBP than those in a higher socioeconomic group. This finding was collaborated by Cois and Erlich in South Africa who reported an inverse relationship between educational status and blood pressure in females [41]. Multiple logistic regression analysis however reveals that the pathway through which SES impacts on the myocardium might be true obesity and hypertension.Concentric remodeling was the most prevalent LV geometric pattern in the pre-hypertensive population. This was in contrast to the findings by Saidu et al [25] and Li et al [29] who both reported concentric remodeling as the predominant LV geometric pattern among pre-hypertensives. The type of LV geometric pattern is determined to a great extent by the prevailing type of stressor on the myocardium, volume or pressure overload [42, 43]. Previous publications had highlighted the impact of hypertension on the LVM and geometric pattern [44, 45] but the effect of pre-hypertension on the myocardium has been largely unevaluated. Pre-hypertension has however been associated with accelerated development of LVH and diastolic dysfunction [46]. Also in the Framingham Heart Study, pre-hypertension was associated with increased incidence of myocardial infarction, stroke, hospitalization for heart failure and cardiovascular death [47]. Concentric remodeling and concentric hypertrophy may predominate in the early stages of the disease process due to the predominating pressure overload whereas eccentric hypertrophy progressively takes over with increased left ventricular mass due to increased volume overload [48]. It has been found that concentric hypertrophy appears to carry the highest risk followed by eccentric hypertrophy, concentric remodeling and normal geometry [49]. In our study, we observed that though there were variation in LV geometric pattern with respect to gender, this was not statistically significant. This was similar to the finding by Maisha et al [50]. This was however in contrast to the observation of Krumholz et al who reported gender-related differences in LV geometric pattern [51]. They however excluded from their study population subjects with diastolic hypertension which might explain the variation in observations.There was a progressive increase in the mean SBP and DBP in patients with normal geometry to patients with concentric remodeling, concentric hypertrophy, followed by a decline in patients with eccentric hypertrophy. A similar observation was made by Silangei et al [26]. The reason for this is not far-fetched as pressure overload tend to predominate at the stages of concentric remodeling and concentric hypertrophy. With time however, the hypertrophied myocardium outstrips is coronary reserve leading to relative hypoxaemia, and apoptotic changes occurring in the myocardium. To overcome the wall stress occasioned by volume overload the chambers dilate with resultant decrease in SBP, DBP, and ultimately eccentric hypertrophy [26]. The most common pattern of LV geometry in our group of obese subjects was concentric LV hypertrophy. This was similar to the observation by Avelar et al [52] but in contrast to other investigators who reported eccentric hypertrophy as the predominant LV geometric pattern amongst obese subjects [52, 53]. The predominance of concentric LV hypertrophy suggests that sympathetic activation, elevated blood pressure could have contributed to the hypertrophy. This study showed that impaired LV diastolic dysfunction occurred earlier than systolic dysfunction with an associated greater atrial contribution to LV filling. We also found that the hypertensive and pre-hypertensive groups had lower E wave and E/A, but higher A wave than the normotensive group. Hence, subjects with pre-hypertension already had LV diastolic abnormalities despite apparently normal systolic function. A similar finding was reported by other investigators [46, 54] who also reported diastolic dysfunction among patients in the early stages of hypertensive heart disease.We also reported that DBP, SBP, and waist circumference correlated with LV remodeling. Result from multivariate logistic regression showed that only DBP was predictive of LV remodeling. This finding emphasizes the need to treat our hypertensive patients to goal and be more proactive in optimizing the blood pressure of our pre-hypertensive subjects.
5. Conclusions
- The findings of this study strengthen the case that socioeconomic status in addition to traditional cardiovascular risk factors has a role to play in the prevalence of LVH. This effect might revolve around its association with hypertension and central obesity. The study also showed that type of LV geometric pattern was dependent on the BP phenotype. In this study we also reported that concentric remodeling was the most prevalent geometric pattern among the pre-hypertension group. Furthermore, we found that LV diastolic dysfunction was already present in this asymptomatic hypertensive and pre-hypertensive population. Finally, despite the fact that the population is undergoing some epidemiological transition, socioeconomic inequalities still persist and its effect on blood pressure and on the myocardium mostly overlooked should be of concern to the clinician.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML