-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2016; 5(1): 1-7
doi:10.5923/j.ijim.20160501.01

Phytochemical Analysis and in-vitro Antimicrobial Activities of Crude Extracts of Aloe barbadensis (Aloevera) Leaf on Microorganisms Isolated from Surgical Wound Patients at Nnamdi Azikiwe University Teaching Hospital (NAUTH), South-Eastern, Nigeria
Ifeanyi Onyema Oshim 1, Reuben Anyi Udeozo Nwobu 2, Uchenna Modestus Ezugwu 1, Evelyn Ukamaka Urama 3
1Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Anambra, Nigeria
2Applied Microbiology and Brewing, Nnamdi Azikiwe University, Awka, Nigeria
3Medical microbiology Option, Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Awka, Nigeria
Correspondence to: Ifeanyi Onyema Oshim , Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Anambra, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Surgical site infection (SSI) is the commonest hospital acquired infection in surgical patients globally. It has remained a major cause of morbidity and mortality and a major source of worry to both the patients, doctors, hospitals and the community as a whole. This present study was aimed to investigate the phytochemical analysis and in-vitro antimicrobial activities of crude ethanol and methanol extracts of the Aloe barbadensis (Aloevera) leaf on five clinical wound isolates (Staphylococcus auerus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Candida albicans). This was a cross-sectional study involving 200 randomly recruited surgical patients between October and November, 2015 at Nnamdi Azikiwe University Teaching Hospital, Nnewi. Pre-tested, interviewer administered questionnaires and laboratory test results were used to collect data, which were analyzed using Statistical Package for Social Sciences, version 20. The antimicrobial activities of the leaf extracts were evaluated using agar diffusion. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal/Fungicidal Concentration (MBC/MFC) of the plant extracts were also determined by agar dilution methods respectively. Two standard strains of Staphylococcus auerus (NCTC6571) and Escherichia coli (NCTC10418) were included, as control organisms respectively. The phytochemical constituents of the leaf extracts were carried out using standard methods. The prevalence of surgical site infection (SSI) was 17.5% (or 35 of 200) among the participants. Staphylococcus aureus was the commonest isolated organism (16 of 35, 46.0%). There was no significant relationship (p < 0.001) between the development of SSI and socio-demographic characteristics. The Aloe barbadensis extracts showed lower antimicrobial activity than the commercially available antibiotics (ciprofloxacin and fluconazole) while Dimethyl sulfoxide (DMSO) was used as a negative control. The efficacy of the extracts towards inhibition of the micro organisms increased with concentrations. Qualitative phytochemical analysis detected the presence of tannins, reducing sugar, saponins and flavonoids in all plant extracts. In conclusion, this leaf extracts could be used as a broad spectrum antibiotics in the treatment of wound infections since it has antimicrobial effects on bacterial and fungal pathogens. Improving on the surgical techniques in the hospital setting is necessary to minimize the rate of hospital acquired infections.
Keywords: Phytochemical analysis, Antimicrobial activity, Aloebarbadensis
Cite this paper: Ifeanyi Onyema Oshim , Reuben Anyi Udeozo Nwobu , Uchenna Modestus Ezugwu , Evelyn Ukamaka Urama , Phytochemical Analysis and in-vitro Antimicrobial Activities of Crude Extracts of Aloe barbadensis (Aloevera) Leaf on Microorganisms Isolated from Surgical Wound Patients at Nnamdi Azikiwe University Teaching Hospital (NAUTH), South-Eastern, Nigeria, International Journal of Internal Medicine, Vol. 5 No. 1, 2016, pp. 1-7. doi: 10.5923/j.ijim.20160501.01.
1. Introduction
- A wound is an abrasion in the skin and the exposure of subcutaneous tissue following the loss of skin integrity which provide moist, warm and nutritious environment that is conducive for microbial colonization and proliferation [1]. Surgical site infection (SSI) is the commonest hospital acquired infection in surgical patients globally [2]. SSI was previously referred to as surgical wound infection but recently it’s been defined as infection occurring within 30 days after a surgical operation (or within one year if an implant is left in place after the procedure) and affecting either the incision or deep tissue at the operation site [2, 3]. The infection could either be superficial or deep, incisional, or involving organs or body spaces. There is increasing emergence of multiple antibiotics resistance of wound isolates which pose enormous public health concerns thus making the need for exploring possible alternatives a necessity. The Aloe vera plant has been known and used for centuries for its health, beauty, medicinal and skin care properties. It consists of a range of compounds including water-soluble and fat-soluble vitamins, minerals, enzymes, mono and polysaccharides, sugar, lignin, phenolic compounds and organic acids [4]. The secondary plant products called photochemical compounds are responsible for the characteristic odours and pungencies and colours of plants and others give a particular plant its culinary, medicinal or poisonous virtues and to aid the survival of the plants [4]. The medicinal qualities of Aloe vera are much diversified and adoptogenic, such as wound healing effect, reduces blood sugar in diabetes, sooths burns, eases intestinal problem, reduces arthritic swelling, ulcer curative object, stimulates immunes response against cancer [5].S. aureus forms a fairly large yellow colony on rich medium and is often hemolytic on blood agar. Staphylococci are facultative anaerobes that grow by aerobic respiration or by fermentation that yields principally lactic acid. The bacteria are catalase-positive and oxidase negative. S. aureus is a major cause of hospital acquired (nosocomial) infection of surgical wounds and infections associated with indwelling medical devices. A few strains are resistant to all clinically useful antibiotics except vancomycin, and vancomycin resistant strains are increasingly-reported. Methicillin resistance is widespread and most methicillin-resistant strains are also multiple drug-resistant [6].P. aeruginosa has become increasingly recognized as an emerging opportunistic pathogen of clinical relevance. Several different epidemiological studies track its occurrence as a nosocomial pathogen and indicate that antibiotic resistance is increasing in clinical isolates [6]. Furthermore, it is constantly reintroduced into the hospital environment on fruits, plants, vegetables, as well by visitors and patients transferred from other facilities. Spread occurs from patient to patient on the hands of hospital personnel, by direct patient contact with contaminated reservoirs, and by the ingestion of contaminated foods and water [7].Theodor Escherich first described E. coli in 1885, as Bacterium coli commune, which he isolated from the feces of newborns. It was later renamed Escherichia coli, and for many years the bacterium was simply considered to be a commensal organism of the large intestine.Over 700 antigenic types (serotypes) of E. coli are recognized based on O, H, and K antigens.Hence, analysis for pathogenic E.coli usually requires that the isolates first be identified as E.colibefore testing for virulence markers [8]. Klebsiella is a genus of Enterobacteriaceae, a frequent cause of nosocomial pediatric infection. Klebsiella can cause infections of the urinary tract, lung, and central venous catheters in high-risk newborns and immunocompromised older children [9]. Klebsiella organisms were named for Edwin Klebs, the noted German bacteriologist [10].Candida albicans cause the superficial fungal infections known as oral thrush, which occurs on the surface of the tongue and inside the mucus of the cheeks. It appears as white patches known as “plaques” which resemble milk curds [11]. Burnt patients are another population at high risk; the wound site is susceptible to colonization by opportunistic fungi such as Candida, but nowadays this is generally well managed and Candidiasis in burnt patients may originate in the gastrointestinal tract or from intravenous catheters [12]. Treatment of circumcision wounds and chronic skin ulcers with locally prepared herbs have been on for generations [13]. Increasing multidrug resistance of pathogens has renewed the research for alternative compounds for the treatment of infectious diseases. Consequently, the present study investigated the in-vitro antimicrobial activities of Aloe barbadensis extracts. This leaf extracts could be used as a spectrum antibiotics in the treatment of wound infections since it has antimicrobial effects on bacterial and fungal pathogens. Improving on the surgical techniques in the hospital setting is necessary to minimize the rate of hospital acquired infections.
2. Materials and Method
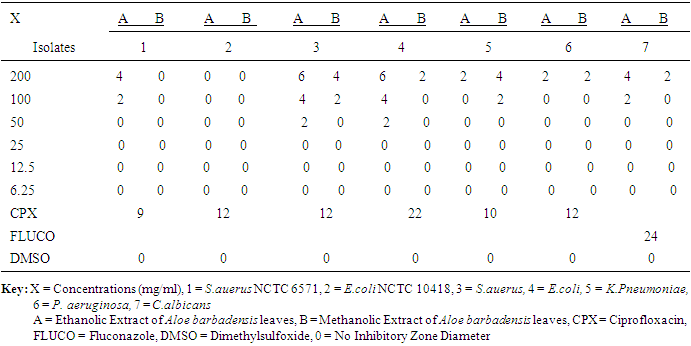
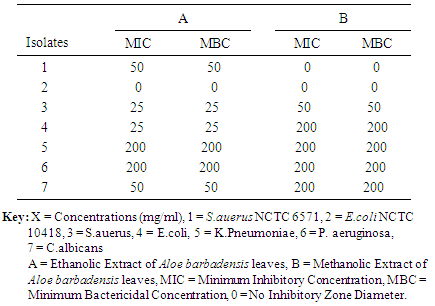
- Study AreaThis study was conducted at NnamdiAzikiwe University Teaching Hospital (NAUTH) Nnewi, Anambra State. A total of two hundred wound swabs were collected from patients in the surgical wards and the subject’s age was between 18-70years.Ethical approval: The study was approved by the institutional ethics committee at Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, Anambra, Nigeria. (the ethical approval number: NAUTH/CS/66/VOL.6/99). The study design in November, 2015.Statistical AnalysisThe results were subjected to statistical analysis using SPSS version 20. Socio-demographic data was analyzed using Chi-square. Level of significant was also considered at P < 0.001. Source and maintenance of test organismsThe wound isolates of Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Candida albicans were obtained from patient’s samples in the surgical wards at NAUTH while Pure cultures of standard strains of Staphylococcus aureus (NCTC 6571) and Escherichia coli (NCTC 10418), (control organisms), were obtained from Department of Pharmaceutical Microbiology and Biotechnology, Nnamdi Azikiwe University, Awka, Nigeria.Collection and identification of plant sampleFresh leaves of Aloe barbadnesis was harvested from farms in Anambra State, Nigeria and identified in the Department of Botany, Nnamdi Azikiwe University, Awka by MrPaulinus Ugwuoke.Preparation of ExtractionAloe barbadnesis leaves were oven-dried at 60°C for 2 weeks and blended into powder and also weighed 200 g of Aloe barbadnesis grounded powder macerated in 95% ethanol and methanol respectively for three days. After maceration, the solution of the plant extracts were filtered through No. 1 What-man filter paper and the resulting solutions dried in a rotary evaporator at 60°C. The dried extracts recovered were placed in sterilized screw-capped bottles and stored at 4°C [14].Phytochemical AnalysisThe phytochemical analysis of methanol and ethanol extract of Aloe barbadnesisleaves (Aloevera leaves) was carried out using standard methods as described by [15].Test for AlkaloidsOne milliliter of the crude extracts of the various plants was mixed with two milliliters of 1% hydrochloric acid (HCl) and heated gently. Mayer’s And Wagner’s reagents were then added to the mixture. Turbidity of the resulting precipitate was taken as evidence for the presence of alkaloids.Test for FlavonoidsTwo milliliters of the crude extracts of the various plants was mixed with few fragments of magnesium ribbon and two drops of concentrated hydrochloric acid (HCl) were added. Pink scarlet colour appeared after few minutes which indicated the presence of flavonoids.Tests for Glycosides (Fehling’s test)Two milliters of a mixture of equal parts of Fehling’s solution 1 and 2 was added to two (2) ml of the crude extracts. The mixture was heated in a water bath for five minutes and white color of precipitate form was noted.Tests for TanninsThe crude extracts of the various plants were mixed with two milliters of 2% solution of FeCl3. A blue-green coloration indicated the presence of tannins.Tests for Saponins (frothing test)Two milliters of the crude extracts was mixed with five milliters of distilled water in a test tube and it was shaken vigorously. The formation of stable foam (frothing) in all the extracts was taken as an indication for the presence of saponins.Tests for PhenolsThe crude extracts of the various plants were mixed with two milliters of 2% solution of FeCl3. The presence of visible coloration indicated the presence of phenol.Tests for reducing sugarsFive milliters of Fehling A and Fehling B reagents were mixed together and two milliters of it was added to two milliters of the crude extracts and gently boiled. The appearance of a brick red precipitate at the bottom of the test tube indicated the presence of reducing sugars.Test for TerpenoidsTwo milliters of each of the crude extracts of the various plants was dissolved in two (2) ml of chloroform and evaporated to dryness. To this, two milliters of concentrated H 2SO 4 was added and heated for about 2 minutes. A grayish colour indicated the presence of terpenoids.Test for QuinonesA few drops of sodium hydroxide was mixed with the plant extract and shaken vigorously.A blue green or red colour indicates the presence of quinines.In-vitro screening of antimicrobial activities of the plant leaf extracts.The agar well diffusion assay method described by [16] was used to evaluate the antibacterial and antifungal activities of the crude extracts of Aloe barbadnesis leaves against the test microorganisms. Dilutions of 100, 50, 25, 12.5, and 6.25mg/mL were prepared from the 200mg/mL stock solution of the plant extracts in a 2-fold dilution process. Twenty (20) mL of molten Mueller Hinton Agar (MHA) and Sabouraud Dextrose Agar (SDA) (for bacterial and fungal isolates respectively) were poured into sterile Petri dishes (90 mm) and allowed to set. Standardized concentrations (McFarland 0.5) of overnight cultures of test isolates were swabbed aseptically on the agar plates and holes (6mm) were made in the agar plates using a sterile metal cork-borer. Twenty (20µl) of the various dilutions of the plant extract and control were put in each hole under aseptic condition, kept at room temperature for one hour to allow the agents to diffuse into the agar medium and incubated accordingly. Ciprofloxacin (5μg/mL) and fluconazole (50µg/mL) were used as positive controls in the antibacterial and antifungal evaluations respectively; while DMSO was used as the negative control. The MHA plates were then incubated at 37°C for 24 hours, and the SDA plates were incubated at room temperature (25-27°C) for 2-3days. The inhibition zones diameters (IZDs) were measured and recorded. The size of the cork borer (6mm) was deducted from the values recorded for the IZDs to get the actual diameter. This procedure was conducted in triplicate and the mean IZDs calculated and recorded.Determination of Minimum Inhibitory Concentration (MIC) of the plant leaf extracts on test isolatesThe Minimum inhibitory concentration (MIC) of the plant extracts on the test isolates were determined by the agar dilution method as described by [17]. The stock solutions (500mg/ml) were further diluted in a 2-fold serial dilution to obtain the following concentrations: 250, 125, 62.5, 31.25, 15.625, 7.8125, 3.91, 1.95, and0.98 mg/mL. Agar plates were prepared by pouring 4 mL of molten double strength MHA and SDA (for bacterial and fungal isolates respectively) into sterile Petri plates containing 1mL of the various dilutions of the extract making the final plate concentrations to become 100, 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78, 0.39, and 0.19 mg/mL.The test isolates which were grown overnight in broth were adjusted to McFarland 0.5 standard and streaked onto the surface of the agar plates containing dilutions of the extract. The MHA plates were then incubated at 37°C for 24 hours and the SDA plates were incubated at room temperature (25-27°C) for 2-3days, after which all plates were observed for growth. The minimum dilution (concentration) of the extracts completely inhibiting the growth of each organism was taken as the MIC. This procedure was conducted in triplicate.Determination of Minimum Bactericidal/Fungicidal Concentrations (MBCs/MFCs) of the plant leaf extracts on test isolatesThe agar portions of MIC that showed no growth were transferred respectively into plates containing freshly prepared MHA and SDA [17]. These plates were incubated at 25-27°C for 2-3 days and were observed daily for growth. The absence of growth at the end of incubation period signifies total cell death. The minimum concentration of the plant extracts that produces total cell death is taken as the MFC.
3. Results
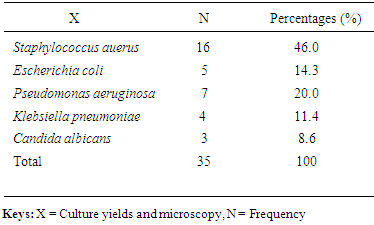
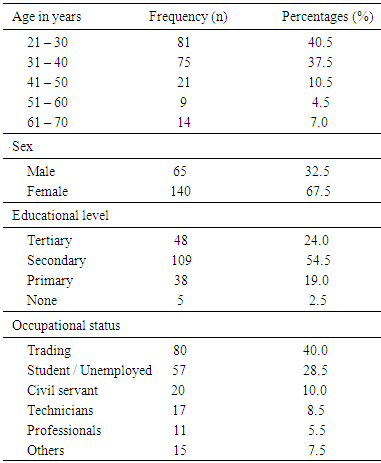
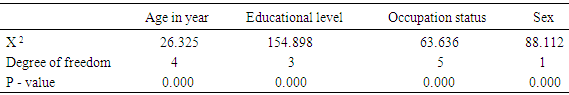
- The prevalence of surgical site infection was 17.5% (or 35 of 200) among the participants while others (165, 82.5%) did not develop SSI. Staphylococcus aureus was the commonest isolated organism (16 of 35, 46.0%) (Table 1). Highest proportion of the participants consisted of those younger than 30 years (81, 40.5%), the least were those in the 50-60 age group (9, 4.5%) (Table 2). Females were more in proportion (135, 67.5%) than males (65, 32.5%) (Tabe 2).
|
|
|
|
|
|
4. Discussion
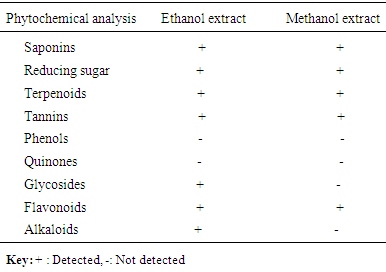
- Surgical site infection (SSI) is the commonest hospital acquired infection in surgical patients globally. Risk factors for SSI may be grouped under the following headings; patient factors, surgeon’s factor, environmental factors and non-surveillance in health facilities. The prevalence of surgical site infection was 17.5% (or 35 of 200) among the participants while others (165, 82.5%) did not develop SSI. Staphylococcus aureus was the commonest isolated organism (16 of 35, 46.0%). Highest proportion of the participants consisted of those younger than 30 years (81, 40.5%), the least were those in the 50-60 age group (9, 4.5%). Females were more in proportion (135, 67.5%) than males (65, 32.5%). A higher percentage of the participants (109, 54.5%) had secondary education, while the least had no formal education (5, 2.5%). Traders were more (80, 40%), while professionals constituted the least (11, 5.5%). The result of the study showed no significant relationship (p< 0.001) between the development of Surgical site infection (SSI) and any of the following factors- age, sex, educational levels and occupation of the subjects. This agreed with the study done by [18], where the relationship was not statistically significant.The reason for the high rate of surgical site infection in this study may be due to poor application of aseptic techniques such as inadequate sterilization of surgical operative equipment, inadequate environmental hygiene and lack of running tap water which sometimes may not be available in the hospital. Qualitative phytochemical analysis detected the presence of tannins, reducing sugar, saponins and flavonoids in all plant extracts. Secondary metabolites of plants such as quinones, glycosides, saponins, flavonoids and all other active principles of plants have been shown to be responsible for the antimicrobial activities shown by these extract.Results obtained from this study, indicated that the Aloe barbadensis extracts showed lower antimicrobial activity than the commercially available antibiotics used which is not in line with the work of [19] who reported that Aloe barbadensis extracts exhibited the strongest antimicrobial activity than the commercially available antibiotics. This is possibly due to the failure of the active ingredient to dissolve in it and all the sensitive extracts were more at higher concentrations than lower concentration. Also, the comparsion of the activity of the plant extract with conventional antibiotics, such as ciprofloxacin and fluconazole confirmed reports by other researchers [20] that constitutional antibiotics are more active than plant extracts. The above findings pointed out that the higher the concentrations of the extracts, the higher the sensitivities of bacterial and fungal isolates to the extracts as showed by the increased size of inhibition zones diameter and this is in conformity with [21] and [22].The comparsion of MIC and MCB of the ethanol and methanol extract of the plant leaf observed that ethanol extract showed greater antimicrobial activity compared to its corresponding extract in the methanolic extract. The ethanolic extract showed the highest activity against the clinical isolates of Staphylococcus aueru sand Escherichia coli then, C.albicans, followed by Klebsiellapneumoniae and Pseudomonas aeruginosa.The prevalence of surgical site infection was 17.5% (or 35 of 200). Staphylococcusaureus was the commonest implicated pathogen in the wound infections. The wound related risk factors associated with Surgical site infection (SSI) were not due to the following factors- age, sex, educational levels and occupation of the subjects but improving on the surgical techniques in the hospital setting is necessary to minimize the rate of hospital acquire infections, in conclusion this plant leaf extracts could be used as a spectrum antibiotics in the treatment of wound infections since it has antimicrobial activity on the bacterial and yeast isolates.Secondary metabolites of this plant extracts which contain tannins, reducing sugar, terpenoids, glycosides, alkaloids and saponins could enhance rapid healing of wound infections.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML