-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2015; 4(2): 17-25
doi:10.5923/j.ijim.20150402.01
Health Care Resource Utilization and Costs in Individuals with Atrial Fibrillation in United Arab Emirates and Kingdom of Saudi Arabia: A Retrospective Cohort Study
Karissa M. Johnston 1, Katherine M. Osenenko 1, Lara Qatami 2, Bonnie M. Korenblat Donato 3, AA Alsheikh-Ali 4, AS Binbrek 5, AS Hersi 6, J. Mould 7, Adrian R. Levy 8
1ICON Epidemiology, Vancouver, Canada
2Bristol-Myers Squibb, Dubai UAE
3Bristol-Myers Squibb, Wallingford USA
4Sheikh Khalifa Medical City, Abu Dhabi, UAE
5Rashid Hospital, Dubai, UAE
6King Saud University Medical City, Riyadh, Saudi Arabia
7Pfizer, New York, USA
8Dalhousie University, Halifax, Canada
Correspondence to: Karissa M. Johnston , ICON Epidemiology, Vancouver, Canada.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Aims: Until recently, persons afflicted with atrial fibrillation (AF) were treated with warfarin to reduce the risk of stroke. Novel oral anticoagulants (NOACs) offer the possibility of less monitoring than warfarin. The objective of this study was to characterize resource utilization and corresponding costs incurred by individuals with AF in a real-world observational setting, in United Arab Emirates (UAE) and Kingdom of Saudi Arabia (KSA). Methods: A retrospective chart review was conducted at three hospitals in UAE and three in KSA to identify AF patients first diagnosed between January 2005 and June 2010. Patient charts were sampled consecutively backwards by diagnosis date, from June 2010 until the target sample size was reached. AF was identified based on ICD-9 code (427.31), from a sample of charts indicating a history of anticoagulant use. Data were collected on hospitalizations, outpatient and emergency room visits, medications, and monitoring and were abstracted from date of first diagnosis until June 2012. Those items were multiplied by country-specific unit costs taken from a public, third party payer perspective, and summed to derive the total annual costs per patient attributable to AF. Costs were converted to, and are reported in, $USD 2013. Results: Among 157 patients in UAE and 152 in KSA, the majority were diagnosed with chronic (persistent or permanent) AF (81% in UAE, 64% in KSA). The mean total annual costs per patient attributable to AF were $1,151 (standard deviation (SD): $1,796) per person in the UAE and $3,001 (SD: $3,502) per person in KSA, with monitoring costs being the largest contributor to costs in both countries (47% and 66%, respectively). In both countries, international normalized ration (INR) was a key contributor to monitoring test costs, and was performed less frequently in patients receiving a NOAC.
Keywords: Atrial fibrillation, Resource use, United Arab Emirates, Kingdom of Saudi Arabia, Treatment
Cite this paper: Karissa M. Johnston , Katherine M. Osenenko , Lara Qatami , Bonnie M. Korenblat Donato , AA Alsheikh-Ali , AS Binbrek , AS Hersi , J. Mould , Adrian R. Levy , Health Care Resource Utilization and Costs in Individuals with Atrial Fibrillation in United Arab Emirates and Kingdom of Saudi Arabia: A Retrospective Cohort Study, International Journal of Internal Medicine, Vol. 4 No. 2, 2015, pp. 17-25. doi: 10.5923/j.ijim.20150402.01.
Article Outline
1. Introduction
- Atrial fibrillation (AF) is the most common cardiac rhythm disorder, accounting for one-third of hospitalizations for cardiac disturbances, [1-3] and associated with increased risk of morbidity and mortality. [4] Individuals with AF have a nearly five-fold increase in the risk of stroke compared to individuals without cardiovascular disease, [5] and often experience substantial decreases in health-related quality of life, which may improve following successful treatment. [6] In 2001, the prevalence of AF was estimated to be between 1.0 and 1.5% in the developed world. [7, 8] Due to an increased risk of AF with increasing age [9, 10] and an aging population, prevalence has increased in recent years and is expected to continue to rise. [2] In the United States, the total number of individuals with AF has been projected to more than double over the next 50 years. [2]Historically, aspirin and warfarin have been the mainstays of treatment for stroke prevention in individuals with AF. [11] While anticoagulation with warfarin is more effective than aspirin in stroke prevention, [12-17] warfarin has been found to be underutilized in practice, due in part to the associated need for frequent monitoring, and the risk of bleeding, particularly in elderly individuals. [18] More recently, novel oral anticoagulants (NOACs), including dabigatran, rivaroxaban, and apixaban, have been introduced into clinical practice. [19] Clinical trial investigators have demonstrated that NOACs have equivalent efficacy and safety to warfarin with less need for ongoing drug monitoring and dose adjustment. [20-22] A recent review found that NOACs present a cost-effective alternative to warfarin. [23]The economic burden of AF is considerable: annual direct medical costs were estimated to be $6.6 billion in in the United States in 2005 [24] and in £459 million in the United Kingdom in 2000. [25] Converted to a common price-year of 2010 Canadian dollars, the annual cost of treating AF was found to be as high as $21,099 in the United States, per patient. [26, 27] Among individuals being treated with warfarin, the costs associated with drug monitoring are an important contributor to overall medical costs. [28]To date, few published data are available describing the burden of AF outside of Europe and North America. [29] Characteristics of these populations differ from those in the Middle East region, with the latter population tending to be younger, with a higher prevalence of obesity, diabetes, and smoking coupled with suboptimal anticoagulation practices and high mortality and stroke rates. [30-32] As a result, local data are required to accurately assess the burden of AF in the region.The objective of this study was to characterize resource utilization and corresponding costs incurred by individuals with AF in a real-world observational setting, in United Arab Emirates (UAE) and Kingdom of Saudi Arabia (KSA). The primary aim of the study was to generate descriptive baseline data describing overall AF-related resource utilization in these two countries, and the study was not powered to statistically compare resource utilization between countries.
2. Methods
2.1. Design and Setting
- A retrospective chart review was undertaken at three large hospitals in the UAE: Rashid Hospital and Dubai Hospital in Dubai, and Sheikh Khalifa Medical City in Abu Dhabi; and at three large hospitals in KSA: King Fahad National Guard Hospital, Prince Sultan Cardiac Centre, and King Saud Hospital, all in Riyadh.
2.2. Subjects
- Eligible individuals included UAE and KSA nationals who received an AF diagnosis between January 1, 2005 and June 30, 2010, identified based on a first ICD-9 code (427.31), from a sample of individuals defined by any history of anticoagulant use. Identified charts were sampled consecutively backwards from June 30, 2010, with a target sample size of 300 charts (150 per country). As the study was intended as descriptive in nature, the target sample size was not based on power calculations, but rather to be sufficient to detect infrequent use of treatments (e.g. off-label use of medication) and adequate precision for characterizing continuous outcomes. With a sample of size 150 per country, a treatment used by 1% of individuals would have a 77.9% chance of being detected, while a treatment used by 5% of individuals would have a 99.9% chance of being detected.
2.3. Data
- From eligible charts, data were collected from AF diagnosis date until June 30, 2012, to allow a minimum potential follow-up of two years. Data were collected on demographic and disease-related characteristics, treatment patterns, and health care resource utilization. AF was classified into paroxysmal, persistent, and long-standing / permanent at time of diagnosis. Those items were multiplied by country-specific unit costs taken from a public, third party payer perspective, and summed to derive the total annual costs per patient attributable to AF. Unit costs of health resources are shown in Appendix Table 1. For the UAE, unit costs were taken from the Dubai Health Authority perspective, the main healthcare regulator and operator of public hospitals in the Emirate of Dubai. For KSA, unit costs were taken from the public Ministry of Health perspective where available (outpatient visits, monitoring tests), and supplemented with private care costs where Ministry of Health costs were unknown (hospitalizations, medications). Costs were converted to 2013 USD using conversion rates current to December 20, 2013 and reported in 2013 $USD.
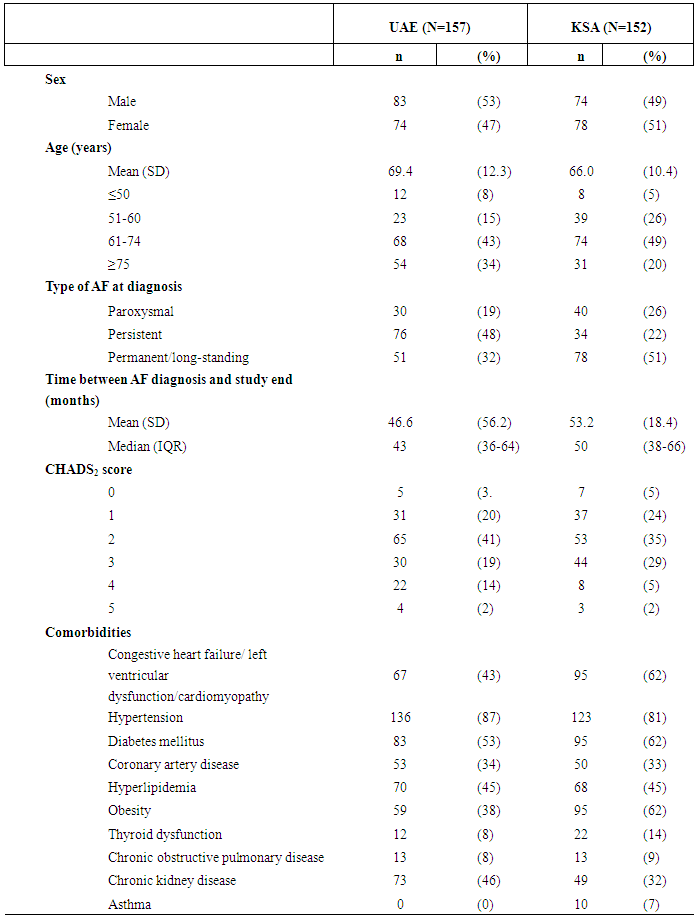 | Table 1. Clinical characteristics of subjects at time of atrial fibrillation (AF) diagnosis in the United Arab Emirates (UAE) and Kingdom of Saudi Arabia (KSA), 2005-2010 |
2.4. Analysis
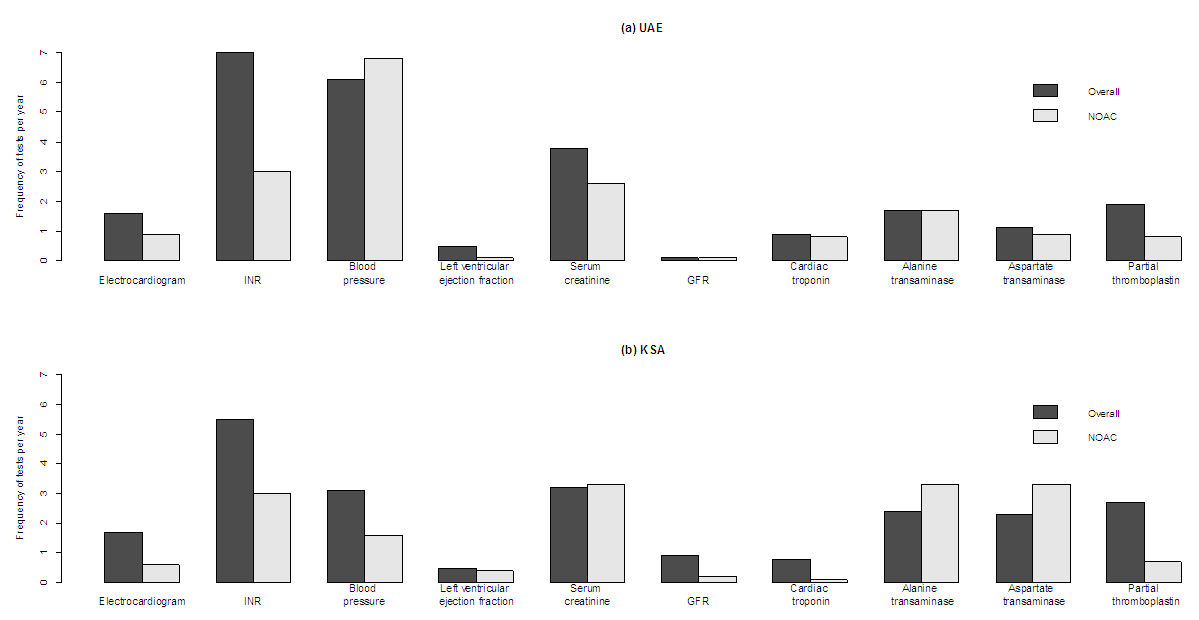
- Categorical variables were characterized by numbers and percent. Continuous variables were characterized by mean, median, standard deviation (SD), and interquartile range (IQR). Treatments were characterized by specific treatment regimens received, and were categorized into the following groups: warfarin, aspirin, clopidogrel, intravenous or subcutaneous anticoagulants (enoxaparin, heparin, fondaparinux), NOACs (dabigatran, rivaroxaban), rate control medications including beta blockers and calcium channel blockers (digoxin, bisoprolol, diltiazem, atenolol, metoprolol, carvedilol, verapamil), and antiarrhythmic medications (amiodarone, propafenone, flecainide). Resource utilization data were analysed using recommended methods, [33] adapted to the setting of a retrospective chart review as has been done previously. [34] Resource utilization was characterized by the proportion of patients who utilized individual categories of resources, and the levels of utilization observed, with annual resource utilization calculated by dividing total resource use by total years’ follow-up time. Annual frequencies of monitoring tests were reported for both the overall sample, and for the subset of person-time during which individuals received a NOAC. This was only possible for tests for which the date of test was recorded (electrocardiogram, international normalized ratio, blood pressure, left ventricular ejection fraction, serum creatinine, glomerular filtration rate, cardiac troponin, alanine transaminase, aspartate transaminase, partial thromboplastin); for complete blood count, thyroid function, serum electrolytes, and echocardiogram, only the total number of tests received across the entire period was available, and a comparison between time receiving a NOAC and overall follow-up time could not be made. Because monitoring test data could not comprehensively be classified based on person-time receiving a NOAC, the analysis was considered to be exploratory in nature, in order to suggest areas for future research, rather than a formal quantitative comparison.For the duration and cost of hospitalizations, medians and interquartile ranges are reported rather than means and standard deviations, because a small number of outliers with long hospitalizations in the UAE led to a skewed distribution.A priori insight into the medical system in UAE and KSA by study investigators with expertise practicing in these countries indicated differences in practice patterns, such that data were analyzed separately by country.
3. Results
- Patient demographics and clinical characteristics are described in Table 1. At participating sites, 157 eligible individuals were identified in the UAE, and 152 in KSA. The median follow up after AF diagnosis was 43.5 months in the UAE and 49.8 months in KSA. In both countries, approximately half the sample was male (52.9% in the UAE and 48.7% in KSA). The mean age at diagnosis was 69.4 years in the UAE (standard deviation [SD] = 12.3) and 66.0 years in KSA (SD=10.4). In the UAE, the most common form of AF was persistent (48.4%), while in KSA it was permanent/long-standing (51.3%), defined as according to American College of Cardiology guidelines. [1] In both countries, the most common CHADS2 score was 2; and the majority had a CHADS2 score of 2 or higher (77% in UAE and 71% in KSA). The most frequent comorbidities were hypertension (81% in both the UAE and in KSA) and diabetes mellitus (53% in the UAE and 62% in KSA). In KSA, obesity, defined as a body mass index greater than 25, and the combined outcome of congestive heart failure / left ventricular dysfunction / cardiomyopathy were each respectively present in 62% of individuals. Unit costs of medications tended to be higher in the UAE than KSA, while unit costs of monitoring tests and outpatient visits were similar or higher in KSA than the UAE. The cost of a general ward day was more than 6-fold higher in KSA, while the cost of an intensive care unit day was more than twice as high in the UAE.The distributions of the most common treatment regimens received and corresponding costs are given in Table 2. Percentages sum to more than 100%, reflecting the fact that a number of individuals received multiple distinct treatment regimens over the course of follow up. In the UAE, warfarin monotherapy was the most commonly prescribed treatment regimen, received by 60% of individuals. In KSA, there was qualitatively greater variability was observed across treatment regimens received: 41% of individuals received warfarin plus aspirin plus a rate control medication, 38% of individuals received warfarin plus a rate control medication, 32% of individuals received aspirin plus a rate control medication, and 18% of individuals received only a rate control medication. The use of NOACs was relatively infrequent in both countries, but more common in KSA, where 16% received any combination including a NOAC, compared to 8% in the UAE. In the UAE, mean monthly medication costs per person ranged from $4.20 for aspirin monotherapy to $301.30 for warfarin plus aspirin plus a rate control medication. In KSA, mean monthly medication costs ranged from $6.50 for rate control medication monotherapy to $591.60 for an intravenous anticoagulant plus aspirin plus a rate control medication. In both countries, the most common medication regimens, including all those prescribed to 10% or more of the population, were associated with a mean cost less than $25 per person per month.
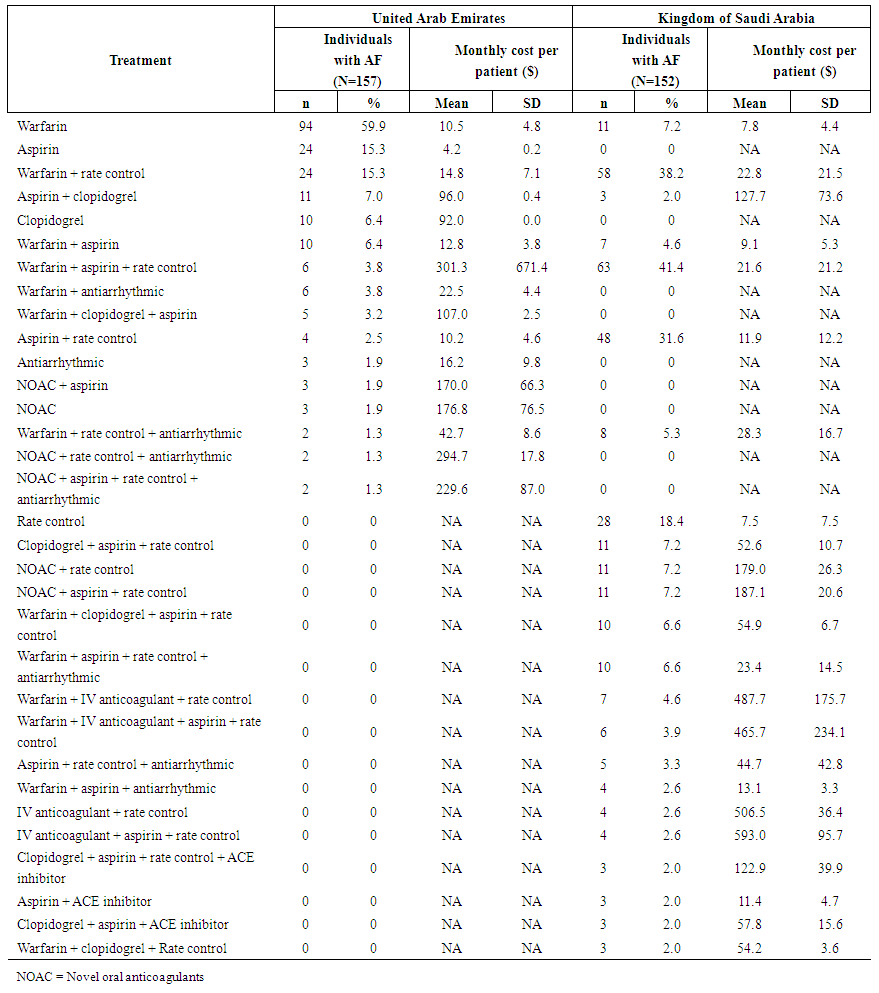 | Table 2. Treatment patterns and costs (2013 $USD) associated with management of atrial fibrillation (AF) management in the United Arab Emirates and Kingdom of Saudi Arabia, 2005-13 |
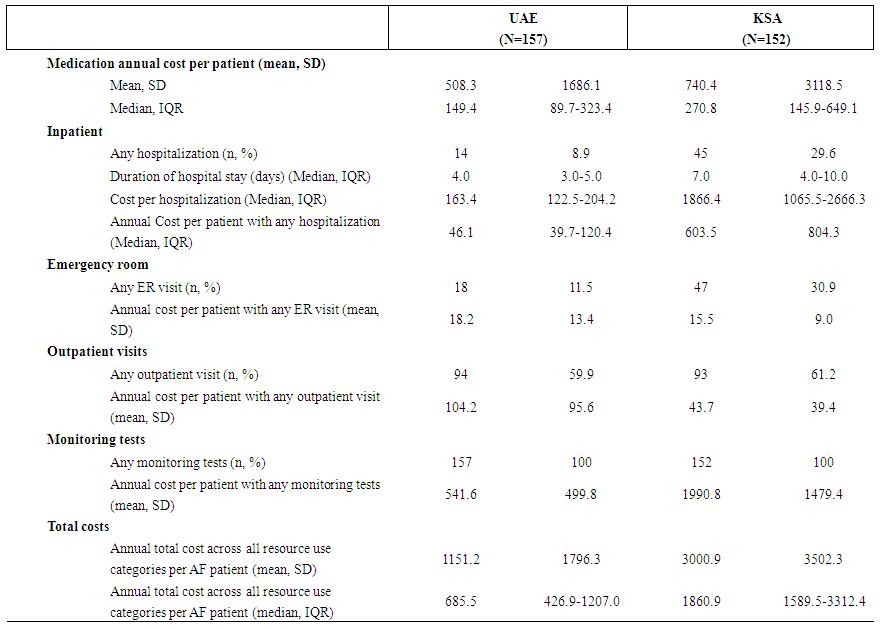 | Table 3. Resource utilization and costs (2013 $USD) for subjects with atrial fibrillation (AF) in the United Arab Emirates (UAE) and Kingdom of Saudi Arabia (KSA), 2005-2013 |
4. Discussion
- In this study, we describe real-world treatment and resource utilization patterns in individuals diagnosed with AF at six large hospitals in the UAE and KSA. The identified population was relatively young, with a median age at diagnosis of 69 years in UAE and 66 years in KSA; this is consistent with other research, [30-32] in which age of AF patients in the Middle East has been found to be younger than that in the United States, [9] Europe, [10] and Canada. [4] The population described here had a relatively high comorbidity burden: in both countries, a majority of individuals were co-diagnosed with hypertension and diabetes mellitus, and approximately half of individuals were obese. In both countries, a majority of patients received anticoagulation with warfarin, most commonly as a monotherapy in the UAE and in combination with rate control +/- aspirin in KSA. Both resource utilization and corresponding medical costs were higher in KSA relative to the UAE: costs were notably higher in the KSA for hospitalizations, emergency room visits, and monitoring tests, resulting in overall annual costs more than twice as high than the UAE. Outpatient visits were the only element of resource utilization that was more costly in the UAE relative to KSA. Given the retrospective and observational nature of the study design, it is challenging to separate health system differences from population differences in assessing the cause(s) for observed resource utilization and cost differences. Based on baseline characteristics observed in patient charts, individuals with AF in the UAE tended to be older than those in KSA, and diagnosed with AF for a longer period of time, although less likely to have permanent or long-standing disease. The distribution of CHADS2 score and frequency of co-morbidities were similar across countries. Given less severe disease despite longer diagnosis times in UAE, there is some evidence of potentially earlier diagnosis than in KSA, which may contribute to lower resource utilization. In addition, different criteria for hospitalization and variation in unit costs can also contribute to differences in direct medical costs.Medical costs observed here for a Middle Eastern population were relatively low compared to international published data. Overall mean AF-related annual medical costs were estimated to be approximately $3,000 2013 USD in KSA and $1,150 2013 USD in UAE. A recent systematic literature review reported a range of annual costs reported in studies conducted across North America and Europe; converting to a common price-year of 2013 USD, these costs ranged from $1,722 to $22,259. [26] Of the 28 North American and European studies included in the review, more than 80% reported an annual cost higher than that observed in KSA and 100% of costs were higher than that observed in UAE. However, globally the annual cost of AF has been estimated to be $3,600 US. [34] Thus, while observed AF-related costs in the UAE and KSA are low relative to those reported for North America and Europe, they are more closely aligned with a global average that incorporates other non-western countries.During the study period of 2005-2012, use of NOACs was relatively low: over the study period, a combination including a NOAC was received by less than 20% of individuals in KSA and less than 10% in UAE. Given the reduced need for monitoring associated with NOACs relative to warfarin that has been previously observed, [20-22] an exploratory comparison was made between person-time spent receiving a NOAC vs. other therapies. In this study, a general trend of reduced monitoring was observed, particularly for INR testing, which comprised a substantial proportion of overall monitoring costs (Figure 1), providing preliminary evidence in a real-world setting that use of NOACs, while associated with higher medication acquisition costs, may lead to cost offsets related to disease monitoring. However, individual test dates were not reported for complete blood count, thyroid function, serum electrolytes, and echocardiogram, such that rates of receiving these tests could not be categorized into person-time on NOACs vs. other treatments, and an overall comparison could not be made.The study was subject to several additional limitations. The data describe UAE and KSA nationals treated at one of three large hospitals included in each respective country, and as such may not be generalizable to the wider population. Health care services received at alternative facilities may not have been captured in patient charts, although the hospitals selected for study inclusion were those that served as a central point of care for patients, and it is anticipated that the majority of health care received by participants was identified. The patient sampling frame was defined based on ever having received an anticoagulant, which may have excluded patients with a diagnosis of AF who did not ever receive an anticoagulant; however, it is anticipated that a substantial majority of AF-diagnosed patients would have received at least one prescription for an anticoagulant over the course of their disease, limiting the magnitude of any resulting bias. Finally, stroke risk was characterized by CHADS2 score, rather than CHA2DS2-VASc which is recommended as preferable, due to a lack of data regarding vascular disease available in patient charts. [35]The results of this study provide baseline data describing resource utilization and costs prior to and in the early stages of NOAC availability. In the future, it would be of interest to conduct a follow-up study specifically powered and designed to assess differences in all resource utilization costs, including all monitoring tests, for time spent receiving a NOAC relative to time spent receiving warfarin and other therapies. Doing so would provide valuable real-world evidence to inform the characterization of the economic costs and benefits associated with NOACs in AF.
ACKNOWLEDGEMENTS
- KJ and KO were employees of ICON plc at the time of this study, a company contracted by BMS for this work. LQ and BKD are employees of BMS. JM is an employee of Pfizer. AL serves as a consultant to ICON plc.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML