-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2015; 4(1): 1-8
doi:10.5923/j.ijim.20150401.01
Risk of Pulmonary Fibrosis in Egyptian Patients with Chronic Hepatitis-C-Infection
Rabie Fathy Abbas1, Khalid Massoud1, Amin M. Hegazy1, Mohamed Said Abdul Aziz Shehata2
1Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
2Radio-Diagnosis Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
Correspondence to: Rabie Fathy Abbas, Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Hepatitis C virus (HCV) infection is an important public health problem in Egypt. Several reports have documented the association of chronic HCV infection with many extra-hepatic manifestations. Interstitial lung involvement has also been integrated into the list of these manifestations [1-21]. Aim of this study is to evaluate the interstitial pulmonary fibrotic changes via high resolution computed tomography (HRCT)of the chest and pulmonary function tests (PFTs) among Egyptian patients with chronic HCV infection. Patients: After departmental ethics committee approval and patient consent were obtained, 20 patients with chronic HCVinfection without any previous pulmonary diseases were enrolled in this study. Methods: All patients were subjected to: History takingandclinical examination, diagnosis of hepatitis-C-virus (HCV) infection by third generation ELISAtest for detection of HCV antibodies, PCR for HCV RNA, liver function tests, renal function tests, Complete blood count, Arterial Blood Gases (ABGs), serum cryoglobulins, Anti-Nuclear-Antibody (ANA), chest x-ray, abdominal ultrasonography, Pulmonary Function Testes (PFTs), and High Resolution Computed Tomography (HRCT)of the chest. Results: The patients were classified into 2 groups according to High Resolution Computed Tomography(HRCT) results:Group I: included 11 patients with chronic liver disease due to HCV who were positive for interstitial pulmonary fibrotic changes (IPF) in HRCT.Group II: included 9 patients with chronic liver disease due to HCV who were negative forinterstitial pulmonary fibrotic changes (IPF) in HRCT. Interstitial pulmonary fibrotic changes were detected by HRCT in 11 out of 20patients (55%), 10out of these11 patients had a restricted pattern lung disease by the Pulmonary Function Testes (PFTs.). There was insignificant correlation between severity of chronic liver disease and presence of interstitial pulmonary fibrotic changes (IPF). Fivepatients were positivefor serum cryoglobulins (3/11 of group I and 2/9 of group II),withinsignificant difference. Conclusion: Chronic hepatitis Cvirus might cause interstitial pulmonary fibrosis as one of its extra-hepatic manifestations. HCV patients should be evaluated for early detection of IPF.
Keywords: Hepatitis C virus, PFTs, HRCT, IPF
Cite this paper: Rabie Fathy Abbas, Khalid Massoud, Amin M. Hegazy, Mohamed Said Abdul Aziz Shehata, Risk of Pulmonary Fibrosis in Egyptian Patients with Chronic Hepatitis-C-Infection, International Journal of Internal Medicine, Vol. 4 No. 1, 2015, pp. 1-8. doi: 10.5923/j.ijim.20150401.01.
Article Outline
1. Introduction
- The most striking feature of chronic hepatitis-C-virus (HCV) is the risk of persistent infection that progress to liver cirrhosis and hepato-cellular carcinoma [29]. Chronic HCV infection is a major public health problem worldwide, and more than 170 million people are chronically infected with HCV (approximately 3% of the world’s population) [8]. In Egypt, the situation is quite worse because the overall anti-HCV antibody prevalence is 14.7% [10]. According to different studies, 40-74% of patients infected with HCV might develop at least one extra-hepatic Manifestation (EHM) during the course of the disease [6, 7]. Several reports have documented the association of HCV infection with many extra-hepatic manifestations, including essential mixed cryoglobulinemia [30], membranoproliferative glomerulonephritis [18-32], sialadenitis [16], lichen planus. [12], porphyria cutanea tarda [15], cardiomyopathy [2]. Within the last 10 years, interstitial lung involvement has also been integrated into the list of extra-hepatic manifestations of chronic HCV infection [1-21]. Emerging clinical data suggested that chronic HCV infection can lead to many direct and indirect effects on the lung. Japanese investigators measured HCV antibodies in patients with Idiopathic Pulmonary Fibrosis (IPF) and observed higher prevalence of serum antibodies to HCV in patients with IPF than in age-matched control group [4]. Idiopathic Pulmonary Fibrosis (IPF) is a chronic, progressive, fibrosing interstitial lung disease of unknown aetiology characterized by a poor prognosis and no proven effective treatment. IPF is the most common of seven idiopathic interstitial pneumonias classified by the American Thoracic Society\European Respiratory Society (ATS\ERS) consensus in 2002, [5]. Chronic HCV infection has been recently identified as an aetiological agent in IPF. [20]. However, there was little information about the contribution of chronic HCV infection in the development of idiopathic pulmonary fibrosis among Egyptian patients, for this purpose this study was planned to declare this association.
2. Patients and Methods
2.1. Patients
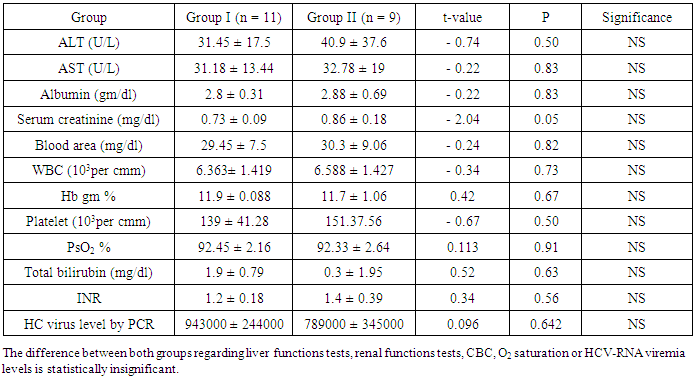
- Twenty patients with chronic hepatitis C virus (HCV) infection; were included in this study. They were selected from inpatients and outpatient clinics of internal medicine department of Sayed Galal Al-Azhar University Hospital. The study was performed during the period from July 2010 to December 2011, after departmental ethical committee approval and patients consent were obtained Patients age ranged between 45-72 years, with mean age is 57.05 ± 10 years, they were 12 males (60%) and 8 females (40%).Inclusion criteria: All the patients had chronic HCV infection (diagnosed on the basis of: elevated liver enzymes [ALT & AST] for at least 6 months, positive serum anti-HCV antibodies and/or positive HCV-RNA by PCR assay and/or liver biopsy consistent with chronic hepatitis.Exclusion criteria: Patients with previous lung disease (prior to the diagnosis of HCV), patients with previous systemic diseases as renal failure, congestive heart failure and collagen tissue disease and chronic liver disease due to another etiology, e. g. Hepatitis-B-Virus (HBV), chronic alcoholism or autoimmune hepatitis patients with a current history or past history of anti-viral treatment for hepatitis C were excluded.Methods: All participants were subjected to:(1) Full history taking and thorough clinical examination. for manifestations of chronic liver disease, liver cell failure, lung disease manifestations and history of drugs intake e.g. interferon.(2) Laboratory investigations that, confirm the diagnosis of HCV infection and exclusion of other causes of chronic hepatitis by assessment of serum HCV-antibodies, Hepatitis–B-Surface Antigen (HBS-Ag) by third-generation enzyme-linked immune-sorbent assay (ELISA) test (Abbotts Diagnostics). detection of Anti-Nuclear-Antibody (ANA) by indirect immune-fluorescence. Confirmation of HCV infections through detection of HCV-RNA by PCR quantitatively. Viral load for patients was measured in international unit (IU), degree of viremia was estimated (according to criteria of the manufacturers, Roche Diagnostics Corporatio Ltd):Viral load<250000 low viremia-viral load> 250000-2500000 moderate viremia-viral load>2500000 high viremia. Liver Profiles: Alanine & Aspartate Aminotransferase (ALT & AST), serum bilirubin, serum albumin, Prothrmbin time (PT), Prothrombin concentration (PC)’ and international normalization ratio (INR). Based on the clinical and liver profile data, patients were stratified according to child-pugh classification [31].● Complete Blood Count (CBC), Kidney functions (blood urea & serum creatinine).● Cryoglobulinemia evaluation by simple blood test, the blood samples were taken very carefully, drawing the blood sample at room temperature then cooling it, to see if the blood precipitates or clumps together [24].● Evaluation of arterial blood gases (ABGs): ABGs samples were taken by percutaneous radial artery puncture in stable seated patients while breathing room air, then, partial pressure of oxygen in arterial blood (PaO2), partial pressure of carbon dioxide in arterial blood (PaCO2) and other parameters were measured with a standard blood gas analyzer.(3) Abdominal ultrasonography:Was performed using, GE LOgic C2-machine to evaluate texture of the liver, portal vein diameter, size of spleen and presence or absence of ascites.(4) Radiological studies: to diagnose interstitial lung disease:[a] Conventional chest X-ray (CXR)[b] High resolution computerized tomography (HRCT) of chest: for evaluation of interstitial pulmonary involvement, the method used was described by Flaherty etal. and Hunninghake etal. [14-17]. HRCT was performed without IV iodinated contrast material so as to prevent lung parenchyma enhancement that may confuse ground glass attenuation (GGA) lesions with normal lung tissue, especially if situated adjacent to areas of structural abnormality, such as emphysema and atelectasis. Reconstructing HRCT images with a high - spatial frequency algorithm (bone algorithm). Enhances the sharpness of lung structural abnormalities by increasing the contrast. Between adjacent structures that have only minor attenuation differences. Traditionally HRCT has been performed in an incremental fashion that limited sampling. To a series of 1-to 2-mm thin sections separated every 10 or 15 mm, excluding 90% or more of the lung parenchyma. Current HRCT using multi-detector helical scanners can provide a volumetric dataset with narrow slice thickness images of the entire lungs obtained during a single breath hold. Contiguous HRCT images make it feasible to confidently distinguish honeycomb and lung cysts from traction bronchiectasis, as well as lung nodules from vascular structures or mucus impaction in small airways. Ideally for the patients: HRCT of the lungs should include supine end-inspiratory, supine. End-expiratory and prone end-inspiratory images.(5) Pulmonary function tests: Forced Vital Capacity (FVC), forced expiratory volume in the first second (FEV1) & FEV1/ FVC ratio were measured and recorded using a spirometer by a trained, experienced chest physicians, spirometry was performed according American Thoracic Society criteria.(6) Statistical analysis of the obtained results (Knapp and Miller, [19]): ● Analysis of the data was done by epi-info, software, version 6.04, word processing and database and statistics program.● The testes used were: Arithmetic mean and standard deviation.● Student's t-test: for testing statistical significant difference between mean values of 2 groups by (P-value) as, P > 0.05 Insignificant, P < 0.05 slight significant, P < 0.02 - P < 0.01 significant, P< 0.002 - P< 0.001 highly significant.● Chi-square test: ficto test for statistical significant relation between different variable and grades in qualitative data. Pearson correlation was used to study the relationship between variables.
3. Results
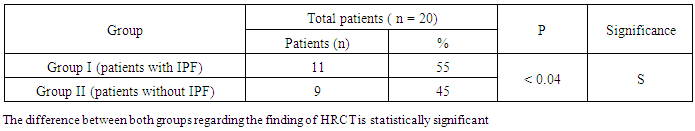
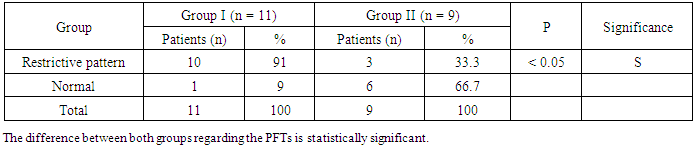
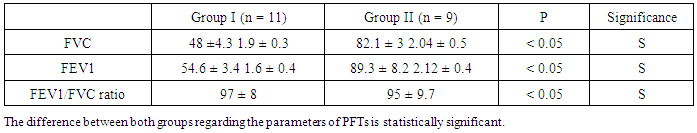
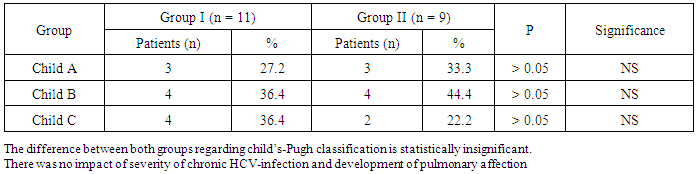
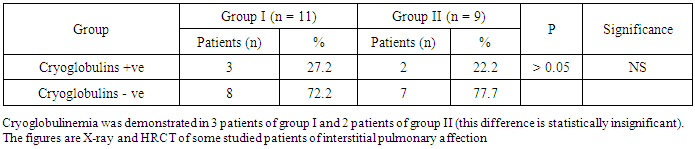
- The results obtained were tabulated and statistically analyzed in tables (1 - 7). The patients were divided into 2 groups according to findings of High Resolution Computed Tomography (HRCT) (table 1):Group I: 11/20 (55%) patients with chronic HCV infection were positive for IPF in HRCT. Group II: 9/20 (45%) patients with chronic HCV infection were negative for IPF in HRCT.Ten out of 11 patients (91%) in group I had restrictive pattern lung disease (P < 0.05, table 3), while 3 patients (33.3%) in group II had restrictive pattern lung disease in pulmonary function tests.No significant correlation was detected between the severity of chronic liver disease (assessed by clinical presentation, examination, laboratory investigations and Child-Pugh scoring) and the presence of IPF among both groups (Table 5, 6).Five patients were +ve for serum cryoglobulins (3/11 of group I and 2/9 of group II), this difference is statistically insignificant (Table 7).
|
|
|
|
|
|
|
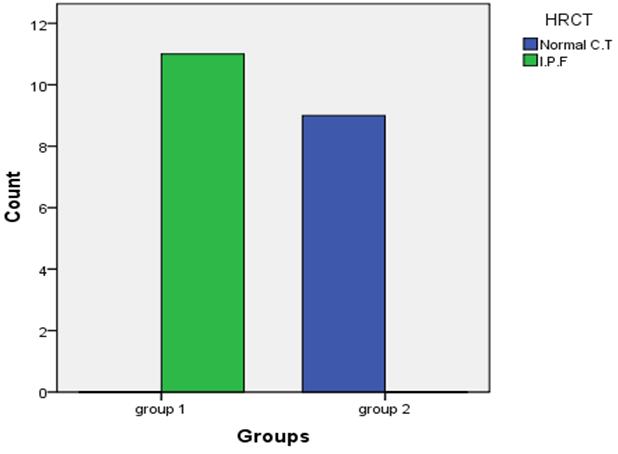 | Figure 1. Findings of HRCT between both groups |
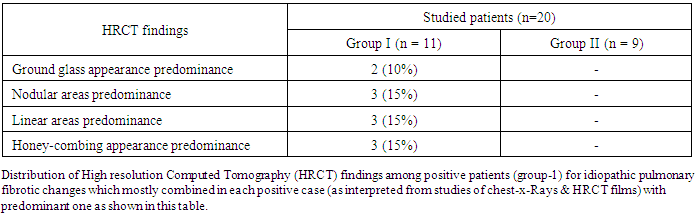
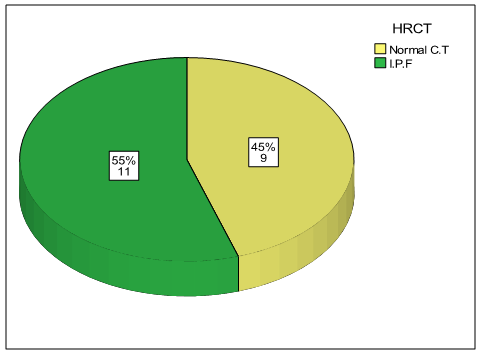 | Figure 2. Finding of HRCT (IPF) among both groups |
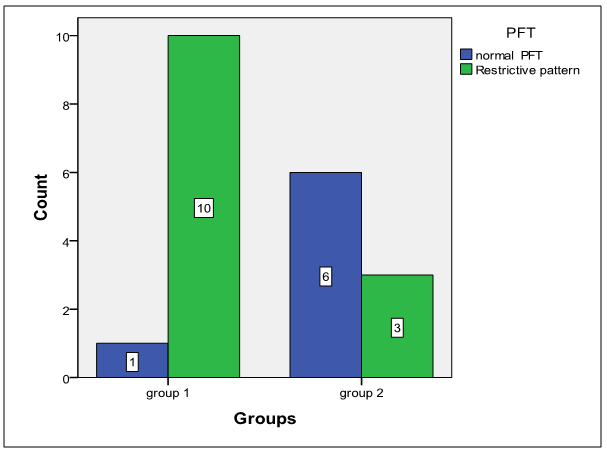 | Figure 3. Showing PFTs results between both groups |
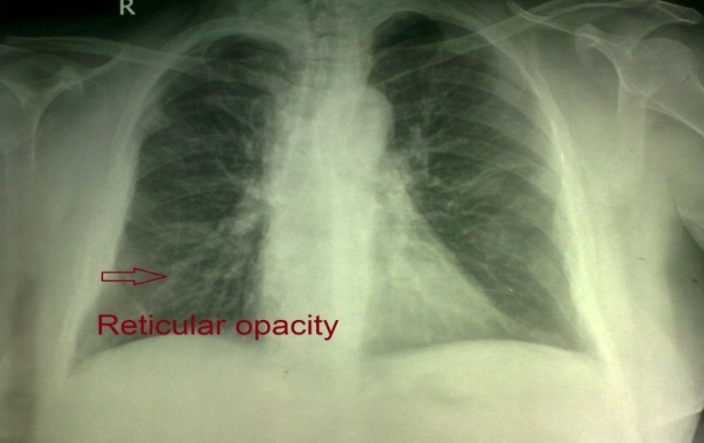 | Figure 4. Chest X-ray of case No. (5): Showing reticular shadowing appearance of IPF |
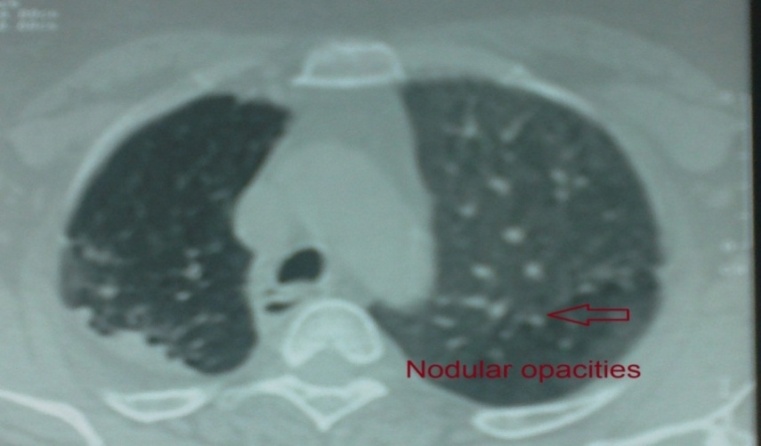 | Figure 5. HRCT - lung window of case No. (12): Showing IPF with predominant nodular shadowing |
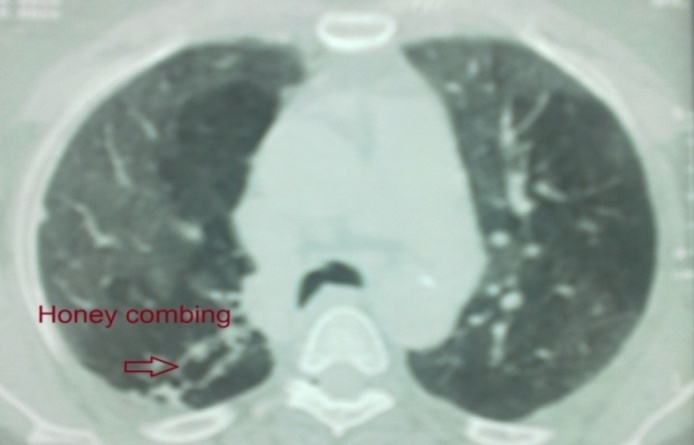
 | Figure 6. HRCT of case No. (9): Showing honeycombing and scarring which is most marked peripherally (IPF) |
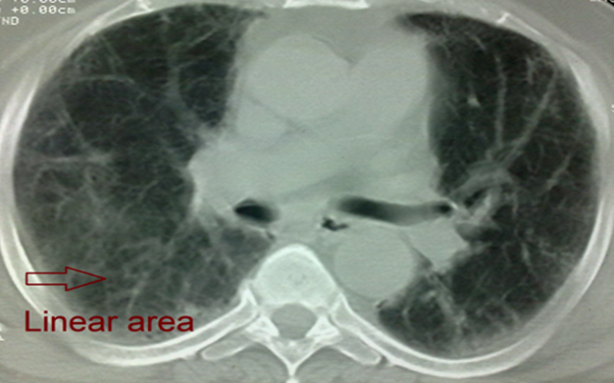
 | Figure 7. HRCT of case No. (14): Showing bi-basal, peripheral predominant reticular abnormality (linear areas) with early traction bronchiectasis and honeycombing (IPF) |
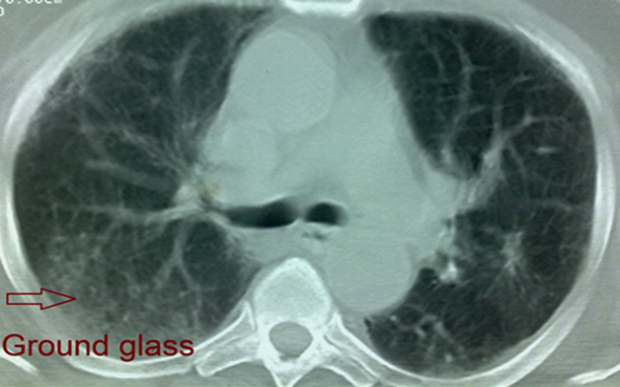 | Figure 8. HRCT scan of Case No. (7): Showing predominant ground glass appearance of IPF |
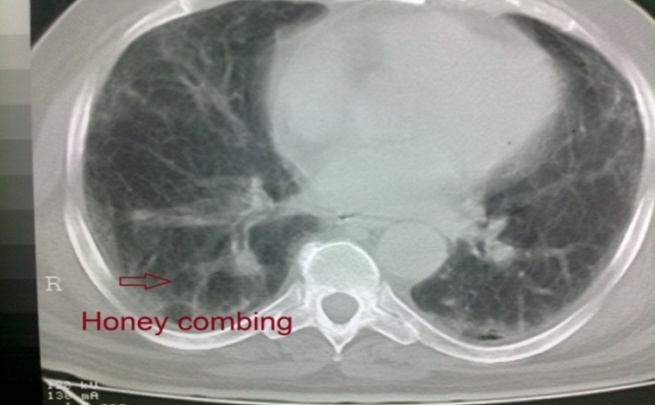
 | Figure 9. HRCT of Case No. (6): Showing bi-basal, peripheral predominant reticular abnormality with traction bronchiectasis and honeycombing (IPF) |
4. Discussion
- Hepatitis C virus (HCV) is a major cause of liver related morbidity and mortality worldwide and represents a major public health problem [3]. Chronic hepatitis C infection has been estimated to be the most common cause of chronic liver disease, cirrhosis and hepatocellular carcinoma [9].Egypt has possibly the highest HCV prevalence in the world; 10 - 20% of the general populations are infected and HCV is the leading cause of chronic liver disease and hepatocellular carcinoma (HCC) in the country [11].HCV is associated with a wide spectrum of clinical and biological extra-hepatic manifestations [26]. In chronically infected patients, the virus can trigger impairment in lymphoproliferation with cryoglobulin production [30] Mixed cryoglobulinemia (MC) with its complications (skin, neurological, renal and hematological) is the most significant extra-hepatic manifestation of HCV infection [13].Over the last decade, an increasing number of reports have suggested that chronic HCV infection is also associated with both direct and indirect effects on pulmonary tissues the direct effect of HCV on the lung may present as worsening of the lung function as initiation or exacerbation of pre-existing asthma and / or COPD., also, an interstitial pneumonitis and / or pulmonary fibrosis [21]. Since HCV is well known to induce chronic inflammation and fibrosis of the liver, it was thought that HCV may play a similar role in the lung and be involved in the pathogenesis of pulmonary fibrosis [23]. HCV-positive patients without clinical pulmonary symptoms have significantly increased epithelial permeability compared with controls, suggesting early interstitial lung disease [4].This work was planned to assess idiopathic interstitial pulmonary fibrosis (IPF) being the most common pulmonary alteration related to HCV in patients with chronic liver disease due to HCV infection by High Resolution Computed Tomography (HRCT) and pulmonary function tests (PFTs)In the present study, 20 patients with chronic liver disease due to HCV infection: 11/20 (55%) of patients were found to have IPF by HRCT (table 1). This result supports the suggestion that HCV infection may cause interstitial pulmonary involvement as one of the extra- hepatic manifestations. This result was in agreement with that documented by Okutan et al. [22] & Erturk et al., [12], who studied the HRCT of the chest of patients with HCV infection, they found that 16/34 (47 %) and 8/21(38%) of their studied patients respectively had IPF findings.As regard to results of PFTs were FVC, FEV1 and FEV1/ FVC ratio in both groups, it was revealed that 10/11(91%), 3/9 (33%) patients had restrictive pattern lung disease by pulmonary function tests, respectively (table-3, P < 0.05). There was statistically significant difference between both groups as regard the parameters of PFTs (Table 4, P < 0.05). This is consistent with results of studies done by Okutan et al., [22] & Erturk et al., [12], whom found 17/34 (50%) 12/21 (57.15%) patients respectively had a restrictive lung disease pattern by pulmonary function tests.The current study was observed the relationship between the severity of chronic liver disease (determined by Child-Pugh scoring) and the presence of IPF in the studied patients (tables 5 and 6), that, there was insignificant difference between both groups as regards these points (P > 0.05). These results were in accordance with that concluded by Shaker et al., [27] who reported that there was no correlation between the degree of liver decompensation and the presence of dyspnea, nor interstitial lung changes by HRCT or by pulmonary function testes, which can mean that pulmonary changes are not related to liver inflammatory changes or altered function. This work, showed that mixed cryoglobulinemia (MC) was positive in 3/11 patients (27.2%) of group I which is higher than detected in group II (2/9 patients 22.2%) but this is statistically insignificant (table 7). This was in agreement with the results of study done by Shaker et al., [27] which stated that the frequency of positive mixed cryoglobulinemia was higher than the frequency of negative mixed cryoglobulinemia in patients with IPF. Also, Okutan et.al, [22] & Erturk et al, [12] & Stevanova et al, [28] reported that, the frequency of positive mixed cryoglobulinemia was higher than the frequency of negative mixed cryoglobulinemia in patients with IPF. So, chronic HCV infection can lead to multiple direct and indirect complications related to pulmonary function.The findings of this study were in parallel with that confirmed by Saleh et al., [25] as the comparison of chest findings with HRCT in both cryoglobulins positive patients and cryoglobulins negative patients with HCV showed insignificant difference. Moreover, they reported that the prevalence of mixed cryoglobulinemia was 61.7% in the studied HCV patients. Our result is within the range of the previous estimates for prevalence of mixed cryoglobulinemia in HCV infection, which varies widely from 10% to 70% [8]. The most frequently affected internal organs in patients with HCV infection and mixed cryoglobulinemia (MC) rather than the liver are the peripheral nerves, kidneys, lungs and joints [24]. In comparison of our findings were in agreement with those concluded by Saleh et al., [25] as understanding the respiratory effects of HCV which is considered to be a major health problem in Egypt, this might improve our approach in the treatment of respiratory problems complicating HCV.
5. Conclusions
- Chronic HCV infection might cause IPF and this could be detected by HRCT in patients presenting with mild respiratory symptoms such as shortness of breath and dry cough.HRCT findings of IPF in presence of mild respiratory symptoms might follow for confident of such diagnosis in absence of lung biopsy. Patient with chronic HCV infection should be evaluated for early diagnosis of IPF.
6. Recommendations
- With the advent of a new era of directly acting anti HCV drugs with high expected cure rate. We suggest further studies on a large scale of Chronic. HCV patients with respiratory affection pre and post treatment with these new drugs for more understanding and documentation of the relationship between Chronic. HCV infection and new pulmonary disorders.Physicians should keep in mind that any patient with chronic HCV infection presenting with mild respiratory symptoms such as shortness of breath and dry cough might have IPF.Further studies on large scale patients are needed for more evaluation of the relationship between HCV viremia, liver pathology and pulmonary fibrotic changes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML