-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2014; 3(2): 31-34
doi:10.5923/j.ijim.20140302.02
Calcinosis Cutis Complicated with Septic Arthritis in a Patient with Rheumatoid Arthritis: A Case Report
Balkarli Ayse1, Kaya Arif1, Avci Ali Berkant2, Karatay Cemile Canan3, Ozkan Umit3, Can Beray3, Cobankara Veli1
1Pamukkale University School of Medicine, Division of Rheumatology, Department of internal Medicine
2Akdeniz University School of Medicine, Division of Rheumatology, Department of internal Medicine
3Pamukkale University School of Medicine, Internal Medicine
Correspondence to: Balkarli Ayse, Pamukkale University School of Medicine, Division of Rheumatology, Department of internal Medicine.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Calcinosis cutis is a disease characterized by precipitation of calcium and phosphate salts in the subcutaneous tissue. It is classified in four different groups depending on the etiology. The most common subtype is dystrophic calcification and is usually associated with connective tissue diseases, including systemic sclerosis, dermatomyositis or systemic lupus erythematosus. However, only two cases have been reported in relation to rheumatoid arthritis. A female patient, who had been followed for 20 years because of the diagnosis of rheumatoid arthritis, was admitted to the hospital due to the complaints of redness in her right leg and discharge. Many radiopaque mineral deposits were observed in the bilateral soft tissue of the lower extremity by direct radiography. There was no evidence of other connective tissue diseases upon evaluation for the differential diagnosis of calcinosis cutis. Serum calcium, phosphorus, alkaline phosphatase, and parathyroid hormone were normal. Septic arthritis in the right knee was detected on the fifth day of follow-up. The aim of this study was to present a case of calcinosis cutis in a patient with rheumatoid arthritis that presented with soft tissue infection resulting in right knee septic arthritis.
Keywords: Rheumatoid arthritis, Calcinosis cutis, Septic arthritis
Cite this paper: Balkarli Ayse, Kaya Arif, Avci Ali Berkant, Karatay Cemile Canan, Ozkan Umit, Can Beray, Cobankara Veli, Calcinosis Cutis Complicated with Septic Arthritis in a Patient with Rheumatoid Arthritis: A Case Report, International Journal of Internal Medicine, Vol. 3 No. 2, 2014, pp. 31-34. doi: 10.5923/j.ijim.20140302.02.
1. Introduction
- Deposition of calcium in subcutaneous tissue, also known as calcinosis cutis, can be related to various conditions such as connective tissue disease, chronic kidney failure, hyperparathyroidism, and malignancy. It is divided into four groups depending on the etiology: metastatic, dystrophic, tumoral, and idiopathic. The most common subtype is dystrophic calcinosis, which is usually related to the underlying connective tissue disease (ACTD) [1]. Dystrophic calcinosis is characterized by normal levels of serum, calcium, and phosphorus. Calcium accumulation can lead to recurrent local inflammation and infection complicated with skin ulcers or nodules and subsequently, can lead to joint contractures and muscle atrophy [2].There are two cases of calcinosis cutis related with rheumatoid arthritis (RA) in the literature. One is a case report and the other takes place in a case series of calcinosis cutis associated with connective tissue diseases [3, 4]. We aimed to present a new case of calcinosis cutis associated with skin ulcers and complicated with septic arthritis in a patient with RA.
2. Case
- A 65 year old female patient presented with fever, fatigue and erythema, and discharge in her legs. Patient was followed for rheumatoid arthritis for 20 years but, failed to come to control examinations for the last year. During this period, she was administered methotrexate (15 mg/week), leflunomide (20 mg/day), pantoprazole (40 mg/day) and folic acid (10 mg/week) as a treatment regime. She did not have any concurrent diseases such as diabetes or hypertension, and did not smoke or consume alcohol. Physical examination showed edema, tenderness, and warmness on the pretibial area. In addition to erythema, the tibia, 1/3 distal, and 1/3 middle regions had small ulcerated areas on the cutaneous calcified plaques. Yellow discharge was coming from the ulcerated lesions spontaneously. She was hospitalized and ultrasonography (USG) examination showed a heterogeneous appearance, including septations compatible with a soft tissue infection. Cultures of blood, urine, and cutaneous ulcers were obtained. Empirical antibiotherapy (intravenous ampicillin sulbactam) was administered. Lower limb radiography showed multiple milimetric radiopaque mineral deposits (figure 1).
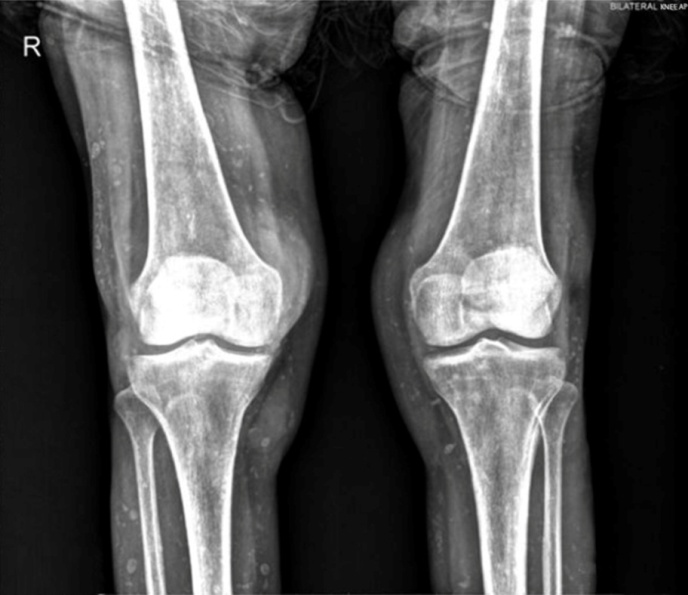 | Figure 1. A bilateral anteroposterior knee radiograph that shows widespread microcalcifications on soft tissue |
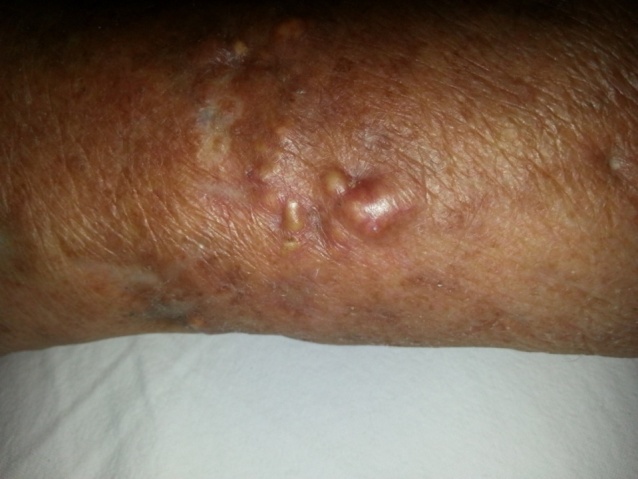 | Figure 2. Pretibial soft tissue calcinosis cutis lesions and skatris after treatment |
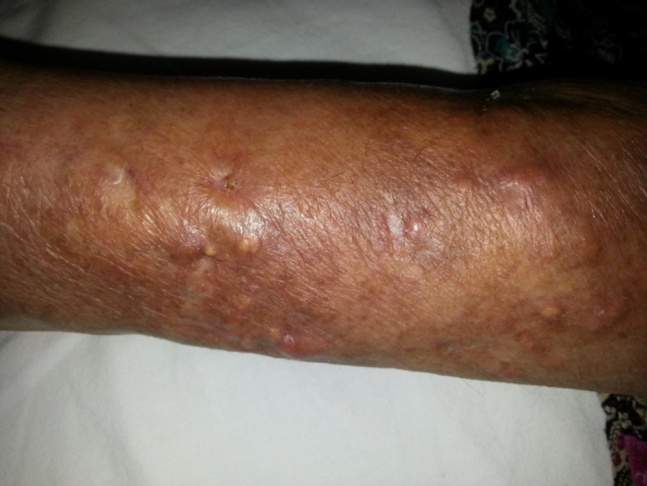 | Figure 3. Pretibial soft tissue calcinosis cutis lesions and skatris after treatment |
3. Discussion
- Calcinosis cutis is divided into four groups metastatic, dystrophic, tumoral, and idiopathic, which depend on the etiology. The metastatic calcification is related to abnormal serum calcium-phosphorus levels and is observed in normal tissues. Moreover, it has been linked to other conditions such as malignancy, hyperparathyroidism, and hypervitaminosis D (2,5). Calcification is usually widespread, and sometimes may be present only on skin and subcutaneous soft tissue. The most common cause of metastatic calcification is chronic kidney disease (CKD). Secondary to CKD, hyperparathyroidism leads to higher levels of serum calcium and phosphate, which cause metastatic calcifications [6]. Tumoral calcinosis has two subtypes: 1) Primary normophosphatemic tumoral calcinosis and 2) primary hyperphosphatemic tumoral calcinosis. Primary normophosphatemic tumoral calcinosis is not familial and serum calcium-phosphorus levels are typically normal. In general, this type of calcinosis has a singular lesion and usually involves trauma. On the other hand, primary hyperphosphatemic tumoral calcinosis is a rare familial disease. In most case, the serum phosphorus levels are high, but calcium levels are normal. In addition, trauma history is not present, and it is characterized by multiple lesions before the second decade. The hyperphosphatemia association is caused by a decrease in renal clearance and an increase in tubular reabsorption [7]. Dystrophic calcification is the most common subtype of calcinosis cutis and is usually associated with ACTD. It is present in normal metabolism and commonly develops in traumatized tissues. In addition to case reports regarding this subject, studies have shown and evaluated the relationship between ACTD and dystrophic calcification. The calcinosis cutis probability is 25% in systemic sclerosis [8], 30-70% in juvenile dermatomyositis [9], and 40% in systemic lupus erythematosus [10]. However, there are only two cases of calcinosis cutis related to RA in the literature [3, 4]. Although normal physiologic tissue concentrations of calcium and phosphate are close to their saturation, tissue calcification is unusual due to the presence of endogenous inhibitors of calcification [11]. Dystrophic calcification etiology is not known; however, several studies were conducted about this subject. One study showed increased calcium binding amino acid and gamma carboxyglutamic acid levels in calcinosis patients [12], while another showed increased IL-1β levels [13]. Kim [14] on the other hand, concluded that apoptosis most likely underlies the mechanism of both physiological and pathological calcification. Interstingly, dystrophic calcifications develop in tissues that change in structure to suitable conditions for calcification, such as hypoxia, trauma, hypovascularity, and tissue structural damage. Genetic predisposition and advanced age can also contribute to this tendency. Presently, there is not a standard pharmacological treatment for prevention or treatment of calcinosis cutis. Warfarin, colchicine, bisphosphonates, or diltiazem can be used either alone or in combination considering the clinical condition of the patient [2]. For small superficial lesions, carbon dioxide laser therapy is an option, but surgical resection may be necessary for larger and more complicated lesions.
4. Conclusions
- Dystrophic calcinosis cutis is observed in connective tissue diseases such as systemic lupus erythematosus, scleroderma, and dermatomyositis. Calcinosis accumulation is known for its role in recurrent local infections and skin ulcerations. Joint contractures may develop secondary to recurrent infection attacks. In the literature, only two cases of calcinosis associated with rheumatoid arthritis have been reported. It should be kept in mind that calcinosis cutis may also be observed in RA patients similar to our case study. In addition, it should be noted that calcinosis accumulation may cause complications such as skin ulceration, soft tissue infections, muscle atrophy, joint contractures, and even septic arthritis. In our case, calcium accumulation caused skin ulcers and soft tissue infection leading to right knee septic arthritis. Besides other connective tissue diseases, RA can be associated with calcinosis cutis and result in severe complications such as septic arthritis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML