-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2014; 3(1): 1-8
doi:10.5923/j.ijim.20140301.01
Protective Effect of Apelin, Amlodipine and Anakinra in Ischemia-Reperfusion Injury in Myocardium
Husam M. Edrees1, 2, Eslam K. Fahmy1, 3, Mostafa H. Abdel-Salam1, 4
1Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
2College of Public Health and Health Informatics, Qassim University, Saudi Arabia
3College of Medicine, Sulaiman Alrajhi Colleges (SRC), Alqassim, Saudi Arabia
4Faculty of Medicine, Tabuk University, Saudi Arabia
Correspondence to: Husam M. Edrees, Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In spite of the advances in the treatment of ischemic heart diseases, acute myocardial infarction (AMI) remains the leading cause of death in the developed countries. Myocardial ischemia reperfusion (I/R) injury occurs during the invasive treatments such as, thrombolysis, angioplasty, coronary by-pass and heart transplantation. The aim of this research is to clarify and compare the protective effects of Apelin, Amlodipine (long-acting dihydropyridine-type (DHP) calcium channel blocker) and Anakinra (Kineret-Interleukin-1 Receptor Antagonist) on ischemia- reperfusion injury in myocardium of experimental animals. Design: A total number of 50 healthy adult male albino rats were used to study and compare the effect of Apelin-13 (injected intraperitoneally at a dose of 1 µg/kg 15 min before reperfusion began), Amlodipine (administered at a dose of 1 mg /kg body weight daily by oral route for 7 days) and Anakinra (a single intravenous shot of 2mg/kg body weight) on preventing myocardial reperfusion injury in experimental animals. Results: The results of this study showed that in Apelin, Amlodipine and Anakinra treated groups, the mean values of all test parameters were significantly lower than ischemia /reperfusion group(p <0.001). In Anakinra treated group, the mean values for all parameters were found to be significantly lower when compared with that Apelin and Amlodipine treated groups. In addition, Infraction mass expressed as percentage of the left ventricle weight was found to be non-significantly changed between Anakinra treated group and Ischemic Control group; moreover, on comparing Apelin with Amlodipine treated groups, no significant changes were found. Conclusion: The results of this study suggest that administration of Apelin, Amlodipine and Anakinra prior to myocardial reperfusion reduces infarct size and markers of infarction in experimental ischemia/reperfusion injury with highest efficacy of Anakinra (Interleukin-1 Receptor Antagonist) more than Apelin and Amlodipine. Thus, they may represent a treatment option in myocardial infarction prior to revascularization.
Keywords: Apelin, Amlodipine, Anakinra Ischemia, Reperfusion
Cite this paper: Husam M. Edrees, Eslam K. Fahmy, Mostafa H. Abdel-Salam, Protective Effect of Apelin, Amlodipine and Anakinra in Ischemia-Reperfusion Injury in Myocardium, International Journal of Internal Medicine, Vol. 3 No. 1, 2014, pp. 1-8. doi: 10.5923/j.ijim.20140301.01.
Article Outline
1. Introduction
- Cardiovascular events are one of the leading causes of death and disability worldwide. In patients with myocardial infarction (MI), the treatment of choice for reducing acute myocardial ischemic injury and limiting MI size is timely and effective myocardial reperfusion using thrombolysis, percutaneous trans luminal coronary angioplasty, and coronary bypass surgery as general treatment strategies of cardiovascular events. However, all of these treatment strategies can cause a myocardial ischemia reperfusion (I/R) injury, which is known to occur on the restoration of coronary blood flow after a period of myocardial infarction [1, 2]. Although it is the only way to save the myocardium from necrotic and apoptotic damages, reperfusion achieved by the restoration of blood flow often aggravates cardiac dysfunction. It is believed that I/R injury is related to the increased oxidative stress and reactive oxygen species (ROS), intracellular calcium overloading, rapid restoration of physiological pH at the time of reperfusion, converge on the MPTP (nonselective channel of the inner mitochondrial membrane), apoptosis and inflammation and the loss of membrane phospholipids especially during the reperfusion. The harmful effects of ROS on cardiac tissue during the I/R can be prevented by endogenous antioxidant systems. Also, the complement system plays a crucial role in the inflammatory events of ischemic injury; thereupon it is important in the pathogenesis of the I/R injury [3]. Polymorphonuclear leukocytes in the reperfusion period are also associated with I/R injury. Therefore these circumstances can increase the irreversible tissue damage. Although sometimes the reperfusion is provided, blood flow cannot be supplied to the myocardial tissue. This is called a no-reflow phenomenon. A lot of exogenous antioxidant agents can be used to prevent this process of injury. Due to these properties of antioxidants, a number of studies have been carried out and have been reported anywhere in the world. These studies demonstrated that these agents can be used in the I/R-induced tissue damage and protect the heart against ROS-related myocardial injury [4].Apelin, as a relatively recently discovered endogenous ligand for the G protein-coupled APJ receptor [5], is not only involved in cardiovascular development, but also plays multiple roles in cardiovascular pathological processes [6]. Studies have shown that apelin acutely protects the heart against ischemia reperfusion (I/R) injury via the activation of phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide (NO) synthase (eNOS) and extracellular signal-regulated kinases (ERK) signaling pathways [7,8] and may have more sustained effects to enhance cardiac performance and improve hemodynamics in heart failure [9,10]. Furthermore, apelin has been shown to stimulate glucose metabolism via the modulation of AMP-activated protein kinase (AMPK) [11], and activation of AMPK has also been shown to improve ER stress [12], which might account for the protection against I/R injury conferred by AMPK [13].Several researchers have described the antioxidant properties of Ca+2 channel blockers as being due to either a direct scavenging effect or the preservation of the superoxide dismutase (SOD) and glutathione peroxidase (GPX) activity. Also Ca+2 channel blockers may inhibit lipid peroxide formation at concentrations present in plasma [14]. Calcium channel blockers, ACE inhibitors and organic nitrates release NO and are useful in the treatment of heart failure. It is reported that amlodipine releases NO from blood vessels which is mediated through the kinins [15-17].Acute total occlusion of a coronary artery is regarded as the underlying cause of acute myocardial infarction (AMI), one of the most common causes of sudden cardiac death in the western world [18]. Recent evidence suggests that innate immune and inflammatory responses play a critical role in the pathophysiology of myocardial ischaemia/reperfusion (I/R). Repression of proinflammatory cytokine and chemokine synthesis is important for alleviation of myocardial ischaemic injury [19, 20]. However, the cellular and molecular mechanisms by which the innate immune and inflammatory responses participate in the pathogenesis of myocardial I/R injury have not been entirely elucidated. The interleukin-1 receptor (IL-1R)-mediated signaling pathway activates several transcription factors such as nuclear factor kappa B (NF-kB), which is an important transcription factor controlling expression of multiple pro-inflammatory genes [21]. Recent studies have demonstrated that Toll-like receptors (TLRs) play a critical role in the induction of innate immune and inflammatory responses [22]. Mammalian TLRs are characterized by extracellular leucine-rich repeat motifs and a cytoplasmic Toll homology domain similar to that of the IL-1R family of proteins, designated as the Toll/IL-1R (TIR) homology domain. The IL-1R/TLR family shares a common signaling pathway leading to NF-kB activation [23].However, a number of emerging therapeutic strategies for preventing lethal myocardial reperfusion injury have shown promise in small proof-of-concept clinical studies, and multicenter randomized clinical trials are currently underway to investigate the effects of these strategies on clinical outcomes [1]. Therefore, the aim of this study is to clarify and compare the protective effects of Apelin, Amlodipine (long-acting dihydropyridine-type (DHP) calcium channel blocker) and Anakinra (Kineret- Interleukin-1 Receptor Antagonist) on ischemia-reperfusion injury in myocardium of experimental animals.
2. Material and Methods
2.1. Experimental Animals
- The current study was carried on 50 male adult albino rats 12–15 weeks old with body weight 180-200 gm, were obtained from the animal house from faculty of veterinary medicine of Zagazig University. Rats were kept in steel wire cages (10/cage) in the physiology research laboratory in faculty of medicine of Zagazig University under hygienic conditions, rats were kept on the normal diet, which consisted of mixed commercial rat laboratory chow and supplied in separate clean containers. Animals had free access to water, kept at room temperature and were maintained on a 12 hr light/dark cycle. All the animals were breed in the animal house and they fed the same type of food to avoid the effect of different food elements on the experiments [24]. The rats were accommodated to laboratory conditions for two weeks before the experiments going on. Rats were numbered and divided into the following groups:Group I: (Ischemic Control group- n = 10): In which the rats were subjected to ischemia for 30 minutes.Group II: (Ischemic group with reperfusion- n = 10): In which the rats were subjected to ischemia for 30 minutes and 120 min reperfusion.Group III: (Apelin Treated group- n = 10): In which the rats were subjected to ischemia for 30 minutes and 120 min reperfusion. This group was injected intraperitoneally with apelin-13 at a dose of 1 µg/kg 15 min before reperfusion began [13].Group IV: (Amlodipine Pre-Treated group- n = 10): In which the rats were given Amlodipine (long-acting dihydropyridine-type (DHP) calcium channel blocker) in a dose of 1 mg /kg body weight daily by oral route for 7 days followed by ischemia for 30 minutes and 120 min reperfusion [15].Group V: (Anakinra Treated group n = 10): In which the rats were administered intravenously with a single shot of Anakinra (Interleukin-1 Receptor Antagonist) in a dose of 2mg/kg body weight 15 min after onset of ischemia. Then the rats were subjected to reperfusion for 120 min [22].
2.2. Drugs and Chemicals
- 1). Amlodipine besylate: 5mg tablets (Pfizer, Norvasc), dissolved in distilled water.2). Apelin-13: Apelin, Phoenix Pharmaceutical, Belmont, CA, USA.3). Anakinra: Kineret, Amgen GmbH, Germany.4). Nitro tetrazolium blue choloride stain (NBT): Bio Basic INC.USA.5). Ethyl carbamate (Urethane): Prolabo, Paris. The drug is available as crystals, which is water soluble.6). Heparin: (1ml ampoule of 5000 I.U. /ml) Nile Co. Egypt.7). Kits for LDH level estimation: Vitro Scient, Egypt.8). Kits for CK-MB level estimation: Pointe Scientific, Inc. USA.9). Kits for malonaldehyde level estimation: Bio diagnostic, Egypt.
2.3. Methods
- The in vivo rat Ischemia/Reperfusion (I/R) injury model has been established in laboratory. Briefly, after anesthesia with urethane (ethyl carbamate) 25% freshly prepared solution in a dose of 1.75-2 gm/kg injected intrapertioneally and endotracheal intubation, animals were ventilated (Animal Respirator DW-2000, Alcott Biotech, Shanghai, China) with room air at 45–60 breaths/min with body temperature maintained at 37°C using a heating pad and monitored with a thermometer. Using sterile surgical procedures, the right carotid artery was cannulated with a 24-gauge angiocatheter for drug infusion. A left thoracotomy was performed through the fourth or fifth intercostal space, and the ribs were gently retracted to expose the heart. After a pericardiotomy, the left coronary artery (LCA) was encircled by a 6-0 prolene suture just distal to its first branch, and its ends were threaded through polyethylene-50 tubing to form a snare for reversible coronary artery occlusion. Before LCA occlusion, animals were anticoagulated (150 U/kg sodium heparin) [13]. Cardiac ischemia was confirmed by an initially pale and later cyanotic area below the suture and ST-T elevation shown in ECG, whereas reperfusion was characterized by the rapid disappearance of cyanosis followed by vascular blush. The duration of ischemia was 30 min, and hearts were examined 2, 12, and 24 h after reperfusion to assess the temporal changes in I/R injury. Ischemia/Reperfusion injury was assessed at 12 or 24 h after reperfusion, the chest was closed in layers at 30 min after reperfusion. Rats were allowed to recover [25].
2.4. Sampling of Blood
- At the end of each experiment blood sample was drawn from carotid artery by syringe connected to arterial cannula, centrifuged at 7000 rotation/min. The serum was separated then stored at -20 c in a dark container until measurement of enzymes (lactate dehydrogenase (LDH), creatine kinase–MB (CK-MB) and malondialdehyde (MDA).
2.5. Determination of Myocardial Infarcted Size
- At the end of the experiments, each heart was rapidly removed and the left ventricle was cut in 1–2-mm-thick circumferential slices following 20 min of incubation at 37°C in 0.1% solution of tetrazolium in phosphate buffer. Stained viable tissue was carefully separated from unstained necrotic tissue and then weighed by an independent observer. The necrotic mass was expressed as a percentage of the total left ventricular mass (index of infraction size) [26].
2.6. Laboratory Analysis
- 1). Estimation of lactate dehydrogenase (LDH): Lactate dehydrogenase was measured according to the method described by Moss et al. [27].2). Estimation of creatine kinase–MB (CK-MB): The enzyme was measured according to the method described by Oliver [28] and modified by Szasz [29].3). Estimation of malondialdehyde (MDA): It was measured according to the method described by Ohkawa et al. [30].
2.7. Statistical Analysis
- In this study due to the small number of rats in each group (N=10) we had a doubt about the use of one way ANOVA as the data may not follow the normal distribution so A one-way ANOVA may yield inaccurate estimates of the P-value when the data are very far from normally distributed. On the other hand the Kruskal–Wallis test does not make assumptions about normality like most non-parametric tests; it is performed on ranked data, so the measurement observations are converted to their ranks in the overall data set [31].So we used the Kruskal–Wallis test to compare the means of all groups and all the data were expressed as Mean ± Standard Deviation (SD), also we expressed the variance, rank mean, median, and percentiles (25th, 50th and 75th) of all study parameters in all groups. Chi square values and corresponding p values were used to detect significance where p values less than 0.05 were considered significant. All parameters were double checked with ANOVA (data not shown).All statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL, United States).
3. Results
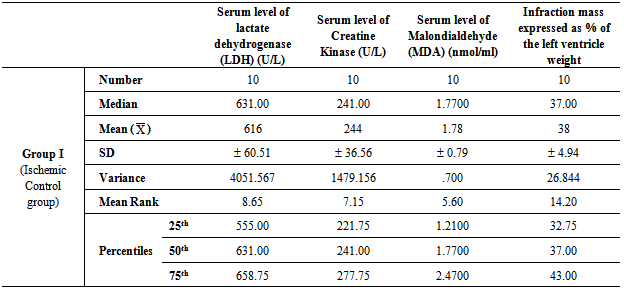
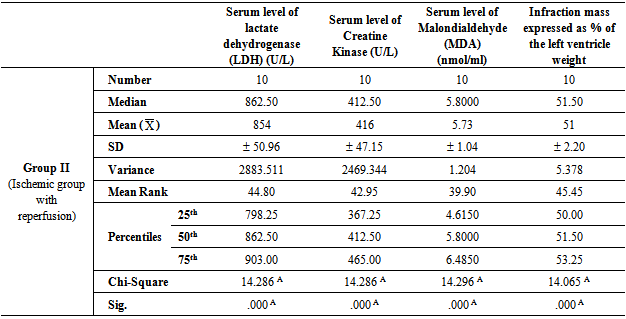
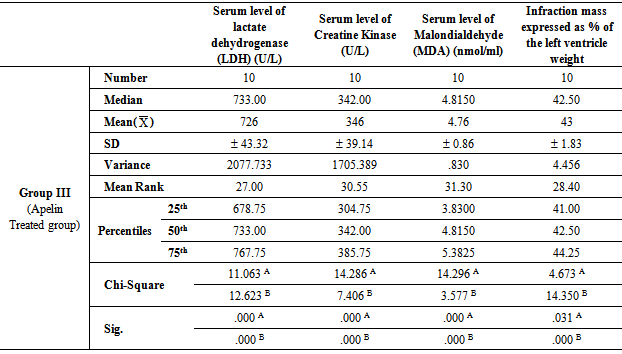
- Table 1, 2, 3, 4 and 5 show serum level of lactate dehydrogenase (LDH) (U/L), serum level of creatine kinase –MB (CK-MB) (U/L), serum level of malondialdehyde (MDA) (nmol/ml) and infraction mass expressed as percentage of the left ventricle weight (index of infraction size) in all studied groups expressed as mean ± standard deviation.In group II (Ischemic group with reperfusion), the mean values were found to be significantly increased (P < 0.001) for all parameters when compared with that of group I (Ischemic Control group). In group III (Apelin Treated group), the mean values were found to be significantly lower (P < 0.001) for all parameters when compared with that of group II (Ischemic group with reperfusion), however, the mean values were significantly higher (P < 0.001) for all parameters except for infraction mass (P < 0.05) in comparison with that of group I (Ischemic Control group).
|
|
|
|
|
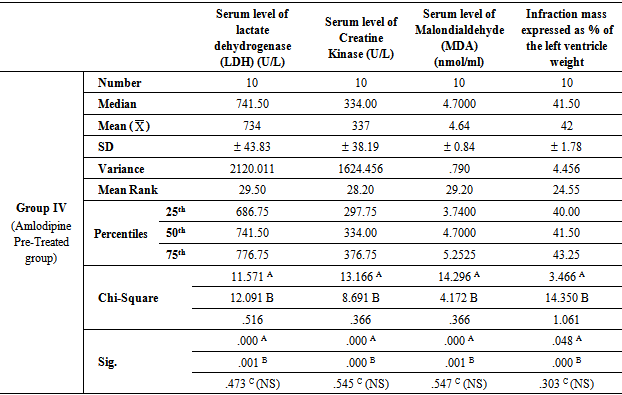
- In group IV (Amlodipine Pre-Treated group), the mean values were found to be significantly lower (P< 0.001) for all parameters when compared with that of group II (Ischemic group with reperfusion), however, the mean values were significantly higher (P< 0.001) for all parameters except for infraction mass (P< 0.05) in comparison with that of group I (Ischemic Control group). On comparing group IV (Amlodipine Pre-Treated group) with group III (Apelin Treated group), the mean values were found to be non-significant for all test parameters.In group V (Interleukin-1 Receptor Antagonist Treated group), the mean values were found to be significantly lower for all parameters when compared with that of group II (Ischemic group with reperfusion), group III (Apelin Treated group) and group IV (Amlodipine Pre-Treated group) (P< 0.001, P< 0.05 and P< 0.05 respectively). However, the mean values were significantly higher in comparison with that of group I (Ischemic Control group) except for infraction mass expressed as percentage of the left ventricle weight (index of infraction size) the mean values were found to be non-significant.
4. Discussion
- Ischaemia-reperfusion injury defined as damage of myocardium following blood restoration after a critical period of coronary occlusion. In fact, ischaemia-reperfusion injury is a clinical problem associated procedures such as thrombolysis; angioplasty and coronary bypass surgery which are commonly used to establish the blood reflow and minimize the damage of the heart due to severe myocardial damage [32]. When myocardial cells are damaged or destroyed due to deficient oxygen supply or glucose, the cardiac membrane becomes permeable or may rupture, resulting in leakage of enzymes. These enzymes enter into the blood stream thus increasing their concentration in the serum. So, serum level of lactate dehydrogenase (LDH) and serum CK-MB are well known markers of myocardial infarction [28]. Oxygen free radicals have been implicated in cardiac ischemic injury which can lead to myocardial infarction, excessive free radicals may be generated, so measurement of malondialdehyde (MDA) is a good marker of radical oxidative stress during reperfusion of the ischemic myocardium [33].In the light of previous data, the present study was designed to clarify and compare the protective effects of Apelin, Amlodipine (long-acting dihydropyridine-type (DHP) calcium channel blocker) and Anakinra (Kineret- Interleukin-1 Receptor Antagonist) on ischemia- reperfusion injury in myocardium of experimental animals as this area of research still not fully explored.The results of the present study revealed that in In group III (Apelin Treated group), the mean values were found to be significantly lower (P< 0.001) for all parameters when compared with that of group II (Ischemic group with reperfusion), however, the mean values were significantly higher for all parameters in comparison with that of group I (Ischemic Control group). These results were in agreement with Tao et al. [13] who tested dose-dependent protection by apelin infusion against I/R injury and revealed that apelin infusion starting 15 min before reperfusion completely abolished Endoplasmic reticulum (ER) stress- dependent apoptosis activation at 2 h of reperfusion and significantly attenuated its activation at 24 h of reperfusion. They demonstrated that apelin exerts its protective effects via PI3K/Akt, ERK, MAPK, and eNOS signaling pathways, with each signaling pathway showing their protective roles against I/R injury, especially in the modulation of ER stress-induced apoptotic activation during the first 24 h of reperfusion. Contradictory results revealed by Rastaldo et al. [26] who mentioned that infarction size (IS) was significantly reduced to 26 ± 4% when apelin-13 was infused after ischemia (Ap-post 0.5). Infarct size in Ap- post- 0.5 µM was also significantly lower than in both Ap-pre-0.5 µM and Ap-pre-1 µM.In addition, The results of the present study revealed that in group IV (Amlodipine Pre-Treated group), the mean values were found to be significantly lower (P< 0.001) for all parameters when compared with that of group II (Ischemic group with reperfusion), however, the mean values were significantly higher for all parameters in comparison with that of group I (Ischemic Control group). On comparing group IV (Amlodipine Pre-Treated group) with group III (Apelin Treated group), the mean values were found to be non-significantly changed for all parameters (P > 0.05). These results were in agreement with Khan et al. [15] who suggested that the protective action of amlodipine, a calcium channel blocker, against the IR induced heart injury in rats is due to increased production of NO and possibly to its antioxidant property. Production of NO in ischemic rat heart and activity of myocardial antioxidant enzymes increased significantly in amlodipine pretreated group that accredits the inhibition of injury in histopathology and electron microscopic studies in amlodipine pretreated group. The mechanism responsible for the release of NO by amlodipine is similar to that of ACE inhibitors that is, modulation of the actions or formation of kinins.In addition, amlodipine reduces myocardial oxygen consumption [34]. It is conceivable that free radicals cause damage at or near the site of their formation. Therefore, as a major source of ROS production, mitochondria could also be the major targets susceptible to ROS attack [35]. The defects in mitochondrial architecture result in decreased activities of mitochondrial enzymes, in the heart, injury induced by IR, thus become a key contributor to intrinsic cell dysfunction [36].Moreover, In group V (Interleukin-1 Receptor Antagonist Treated group), the mean values were found to be significantly lower (P< 0.001) for all parameters when compared with that of group II (Ischemic group with reperfusion), however, the mean values were significantly higher in comparison with that of group I (Ischemic Control group) except for infraction mass expressed as percentage of the left ventricle weight (index of infraction size), the mean values were found to be non-significantly changed (P > 0.05).Moreover, on comparing with group III (Apelin Treated group) and group IV (Amlodipine Pre-Treated group), the mean values were found to be significantly decreased (P< 0.001) for all parameters. These results were in agreement with Grothusen et al. [22] who hypothesized that a one-time intravenous administration of the IL-1 receptor antagonist Anakinra prior to reperfusion may limit myocardial I/R injury. They reported that Anakinra reduces infarct size in this experimental setting. The influx of inflammatory cells into the area of former ischemia contributes to all of these mechanisms. Therefore, modulating the inflammatory reaction during myocardial reperfusion has been an attractive target for experimental as well as clinical trials. IL-1α and β via activation of the IL-1 type I receptor have been indicated to promote a multitude of inflammatory processes. In addition, both cytokines have been implicated in cardiac remodeling and heart failure. Thus, blocking the IL-1 type I receptor by Anakinra seems a promising therapeutic target. A cardioprotective effect of Anakinra has already been proposed by the results of different experimental studies. For example, cardiac overexpression of IL-1 receptor antagonist reduced infarct size in a rat model of myocardial infarction. The underlying mechanisms may involve a decrease in cardiomyocyte apoptosis, as indicated by a study of Abbate et al. [37]. In addition, Anakinra positively influences endothelial dysfunction, reduced oxidative stress, and improved ventricular function in patients with rheumatoid arthritis, a known risk factor for cardiovascular events [38]. one-time application of Anakinra prior to myo-cardial reperfusion leads to a decreased extent of experimental myocardial I/R injury and, therefore, may not only be considered as a treatment option after revascularization but instead may also be beneficial in the very early phase of acute myocardial infarction.Interesting finding of the present study that Anakinra (Kineret-Interleukin-1 Receptor Antagonist) had more protective effect on ischemia-reperfusion injury in myocardium of experimental animals when compared with that of Apelin and Amlodipine (long-acting dihydropyridine- type (DHP) calcium channel blocker). This finding was evidenced by significant decrease of the mean value of serum level of lactate dehydrogenase (LDH), Creatine Kinase, Malondialdehyde (MDA) and infraction mass expressed as percentage of the left ventricle weight in group V (Interleukin-1 Receptor Antagonist Treated group) when compared with that of group III (Apelin Treated group) and group IV (Amlodipine Pre-Treated group) (P< 0.05 and P< 0.05 respectively). This finding suggests that the dominant cause of cardiac myocyte injury following ischemia / reperfusion is related to inflammation and apoptosis rather than intracellular calcium overloading or oxidative stress and reactive oxygen species (ROS).
5. Conclusions
- The present study suggests that apelin, amlodipine (a calcium channel blocker) and Anakinra (Kineret- Interleukin-1 Receptor Antagonist) have cardioprotective effect as post conditioning mimetic as it could reduce the markers of tissue damage and decrease the infarction size with more observable effect for Anakinra (Interleukin-1 Receptor Antagonist). Thus, they may represent a treatment option in myocardial infarction prior to revascularization and therapeutic strategy for protection of myocardium against ischaemic / reperfusion injury.
ACKNOWLEDGMENTS
- The authors would like to acknowledge the supporting team of technicians in the Department of Physiology, Pathology and Medical Biochemistry, Zagazig University for their assistance in the experimental work and laboratory analysis. This research received no specific grant from any funding agency in the public commercial or not-for-profit sectors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML