-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2013; 2(2): 11-15
doi:10.5923/j.ijim.20130202.02
Invasive Fungal Infection Prophylaxis in Immunocompromised Hosts; the Consensus from Shiraz IFI Study Group, December 2012, Shiraz, Iran
Mohsen Moghadami1, Mani Ramzi2, Mehdi Dehghani2, Mohammad Ali Davarpanah1, Habib Nourani Khojasteh2, Reza Vojdani2, Shirin Haghighat2, Amir Roodgari1, Leila Nafarieh3, Mohammad Afarid3, Mohammad Torabi Nami3, 4
1Division of Infectious Disease, Department of Internal Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Hematology and Oncology, Shiraz University of Medical Sciences, Shiraz, Iran
3Behphar Scientific Committee, Behphar Group, Tehran, Iran
4School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence to: Mohammad Torabi Nami, Behphar Scientific Committee, Behphar Group, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Invasive fungal infections (IFIs) are amongst the most important causes of morbidity and mortality in immunocompromised patients. To more effectively approach IFIs both in prophylactic and therapeutic perspectives, one of the crucial imperatives is to know about the local fungal epidemiology. This is relatively lacking in our current setting. Insufficient diagnostic tools as well as the progressive and life-threatening course of IFIs when consolidated, compromise the IFI treatment outcome in many cases. This necessitates primary antifungal prophylactic approaches in immunocompromised hosts who are mainly at risk. To stay aligned with regard to IFI prophylactic strategies in our practice and to pave the path towards definition of local guidelines, the “Shiraz IFI Study Group” held a consensus meeting on 6th December 2012, in Namazi hospital, Shiraz, Iran. Experts from hemato-oncology and infectious disease fields reviewed the available evidence-based data with regard to prophylactic strategies against IFIs so that to draw the preliminary agreed-upon algorithmic approach.
Keywords: Invasive Fungal Infection, Immunocompromised, HSCT, Prophylaxis, Aspergillosis
Cite this paper: Mohsen Moghadami, Mani Ramzi, Mehdi Dehghani, Mohammad Ali Davarpanah, Habib Nourani Khojasteh, Reza Vojdani, Shirin Haghighat, Amir Roodgari, Leila Nafarieh, Mohammad Afarid, Mohammad Torabi Nami, Invasive Fungal Infection Prophylaxis in Immunocompromised Hosts; the Consensus from Shiraz IFI Study Group, December 2012, Shiraz, Iran, International Journal of Internal Medicine, Vol. 2 No. 2, 2013, pp. 11-15. doi: 10.5923/j.ijim.20130202.02.
Article Outline
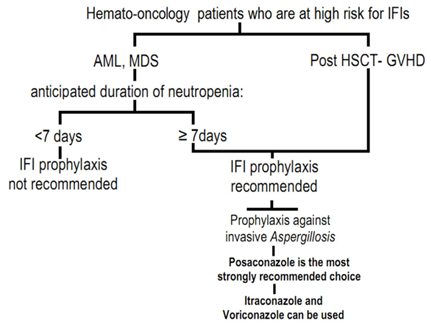
1. Management of Invasive Fungal Infections (IFIs) in Hemato-oncology Patients; the Unmet Needs
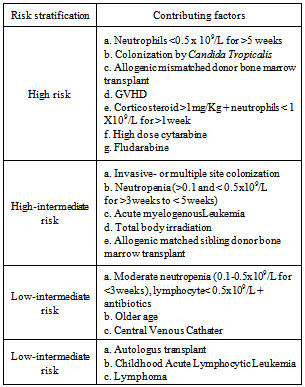
- Invasive Fungal Infections (IFIs) are among the most prevalent causes of morbidity and mortality in hemato-oncology patients. Comprehensive knowledge about the local fungal pathogens epidemiology is a determinant factor to define local prophylactic and therapeutic protocols in IFI management. Global IFI epidemiology data demonstrates that amongst the high risk population[i.e. in patients with acute myelogenousleukemia (AML), myelodysplastic syndrome (MDS), post hematopoetic stem cell – (HSCT) and solid organ transplant recipients], the most prevalent pathogens for IFIs are Aspergillus (29%-57%) and Candida (34%-42%) species.[1] The morbidity and mortality due to invasive aspergillosis has surged over the past years. Some reasons behind this may include: 1-higher number of patients with malignancy are being treated with intensive immunosuppressant therapy regimens; 2-patients have relatively improved survival from the formerly fatal bacterial infections and 3-the number of patients undergoing allogenic-HSCT and organ transplantation is rising.[1, 2]Meanwhile, the diagnostic challenges and the newly emerging fungal subspecies make the timely and proper antifungal drug therapy approaches crucial. The available diagnostic tools are often insufficient for clinical decision making in treating IFIs.[2] With regard to the new subspecies, recent DNA sequencing studies have revealed 30 newly identified sibling species for Aspergillusfumigatus complex. The least estimate is that 5-10% of invasive aspergillosis caused by these sibling species have previously been known as Aspergillusfumigatus. This is clinically relevant since these subspecies would not adequately respond to Aspergillusfumigatus-sensitive azoles.[3]Our local recent clinical experience has shown that the prevalence of invasive zygomycosis with its Rhino-Cerebral Mucormycosis (RCM) presentation seems to be steadily rising. Moreover, the progressive nature of invasive zygomycosisand the limited treatment options make it a challenging clinical encounter.There are reports indicating the emerging challenges in prevention and treatment of IFIs. A recent investigation has revealed that over 90% of the itroconazole-resistant A. fumigatusisolates has already developed a TR/L98H mutation in CYP51A gene. This mutation might have occurred due to the wide environmental use of azoles. Interestingly, 80% of these variants are likewise resistant to voriconazole while 17% to posaconazole.[3]Some established risk factors such as prolonged (≥ 7days) or severe neutropenia, acute or chronic extensive graft-versus-host disease (GVHD), prior IFIs, corticosteroid use, HLA mismatched donors and increased age of HSCT recipients, have shown spectacular contribution to potentially fatal IFIs. Along these lines, some emerging risk factors predispose patients to an increased risk of IFIs. These include cytomegalovirus infection, gancyclovir treatment, respiratory viral infections, lymphopenia and increased bone marrow iron stores. Given these, the compounded multiple risk factors for IFI should be taken into consideration when proceeding to patients’ risk stratification. Table 1 summarizes the risk stratification for IFIs.[1]
|
2. Rationale for Prophylactic Approach to IFIs
- All IFIs are known to contribute as substantial sources for mortality mainly in patients who are stratified as high risk .[2] Moreover, regardless of the patients’ risk class, invasive aspergillosis is associated with a high mortality. Aspergillosis-related case fatality rate in bone marrow transplanted patients (HSCT) exceeds 80%.[4]One of the main unmet needs in IFI management is that when clinical condition mandates antifungal therapy, culture based diagnosis is usually not available. Therefore, in many instances, instead of targeted treatment, empirical and preemptive strategies become warranted.[5, 6]There are some non-culture based diagnostic tools, mainly ELISA (Enzyme-linked immunosorbent assay)-based assays which help detecting fungal cell wall components. These include Galactomannan (GM) and (1,3)-β-D-glucan assays. The above diagnostic tools generally suffer from shortcomings such as false positive/negative results, influenced results following antifungals use and variably reported sensitivities. Beta-D-glucan test is technically challenging and costly, however, to avoid missing distinct high-risk cases, its availability is supported. Further to the above non-culture diagnostic tests, detection of fungal DNA using Polymerase Chain Reaction (PCR)-based assays has been applied in some studies, although not yet validated.[7-9] GM is almost restricted to invasive aspergillosis and is recommended to be done twice so that to have the false-positive rate diminished.[7]Due to the challenges in treating IFIs and that the clinical decision should not be delayed until after consolidation of the life-threatening IFI, prophylactic approaches especially in high-risk patients (i.e. AML, MDS cases who experience prolonged neutropenia and post HSCT patients with GVHD) have gained considerable attention.The most recent update from the National Comprehensive Cancer Network (NCCN) guideline for the prevention and treatment of cancer-related infections[10] has supported the use of various antifungal agents (at different recommendation levels) for IFI prophylaxis in high-risk patients ( Figure 1). As outlined in figure 1, fluconazole has gained preference in IFI prophylaxis amongst ALL, auto- and, allo-HSCT (neutropenic) patients. Meanwhile, itraconazole prophylaxis is restricted to neutropenicallo-HSCT cases. Posaconazole is the only antifungal agent which has gained level 1 recommendation for IFI prophylaxis among neutropenic AML/MDS patients and those who have undergone HSCT and experienced GVHD.[10] Following guideline review and plenary discussions, we agreed upon the prophylactic use of fluconazole in ALL; posaconazole, voriconazole and amphotericin B in neutropenic AML/MDS, fluconazole in auto-HSCT; fluconazole, itraconazole and posaconazole in neutropenicallo-HSCT and posaconazole in significant GVHD.
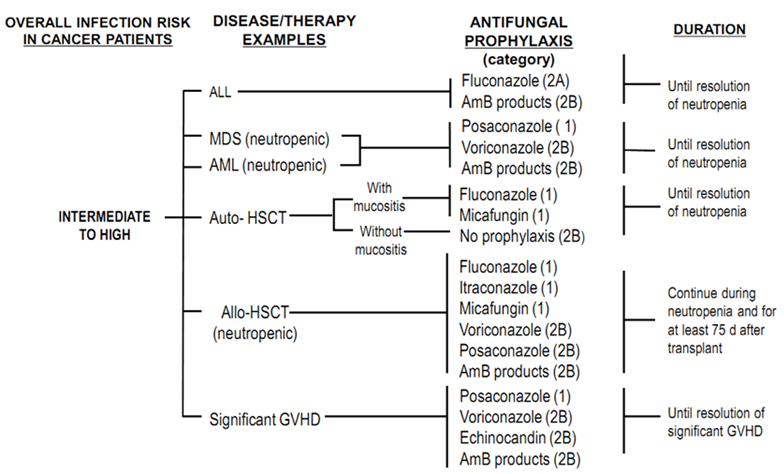 | Figure 1. NCCN guideline has supported the use of various antifungal agents(at different recommendation levels) for IFI prophylaxis in high-risk patients[10] |
3. IFI as a Potential Cause of Death in Allogenic-HSCT; the Position of Prophylactic Azoles
- Clinical evidence has suggested that IFI prophylaxis per se, decreases the risk of complications and mortality in hematologic malignancies and HSCT recipients.[12, 13] Therefore, selecting an effective antifungal not only based on the IFI risk profile but also the affordability issue is an executive clinical decision to be made for each eligible case. In addition to cost, other factors such as long-term tolerability and ease of use play a particular role. Further to the above, the decision to commence primary prophylaxis against IFI should be made in the light of local epidemiology. 2 Where Aspergillus infection predominates in a center, prophylaxis with mould-active azoles should be greatly considered.[10, 11]Moreover, voriconazole has been shown to exert no additional benefit in reducing the incidence of infection or extending survival as compared to itraconazole and fluconazole.[14,15] Nevertheless, recent evidence has supported the superiority of mould-active antifungals (i.e. itraconazole,posaconazole and voriconazole) for prophylaxis.[16] There has been no reported direct comparison between posaconazole and voriconazole in IFI prophylaxis. The most recent European Bone Marrow Transplantation (EBMT) conference received a mixed treatment comparison of randomized clinical trials on IFI primary prophylaxis in allogenic-HSCT recipients[17], suggesting that mould-active azoles are more effective than fluconazole to prevent IFI incidence in this population. This systematic review demonstrated no clear distinction between itraconazole, posaconazole and voriconazole.
4. The Role of Posaconazole in IFI Prophylaxis
- Following the first European Conference on Infections in Leukemia (ECIL) meeting, posaconazole acquired the A1 recommendation for antifungal prophylaxis in AML/MDS patients and in allogeneic HSCT recipients during acute (grade II or more) or extensive chronic GVHD.[18] Posaconazole is an extended-spectrum triazole antifungal agent[19-21] which possesses activity against a wide range of yeasts and moulds, including Candida species (such as C. glabrata).[20] Posaconazole’s in vitro activity is shown to be generally greater than that of fluconazole and either equal to or greater than that of itraconazole, voriconazole, and amphotericin B.[20, 21]Two key randomized studies have shed light to the acknowledged role of posaconazole in IFI prophylaxis in immunocompromised (AML/ MDS with prolonged neutropenia) and post HSCT-GVHD hosts.[12,13] The first study enrolled 602 newly diagnosed or relapsed AML or MDS patients who were treated with intensive chemotherapy. Patients received posaconazole, 200 mg tid (n=304) or a standard azole regimen[either fluconazole, 400 mg qd (n= 240) or itraconazole, 200 mg bid (n=58)] with each cycle of chemotherapy until remission or for up to 12 weeks. Posaconazole treatment was associated with fewer total IFI during the treatment phase (2% vs. 8%; P= 0.0009) and fewer infections owing to Aspergillus (1% vs. 7%; P= 0.0001).[12]Analysis of death rate within 100 dayspost-randomization demonstrated a survival benefit in favour of posaconazole in terms of all-cause (15% vs. 22%; P = 0.03) and IFI-related mortality (2% vs. 5%; P =0.0209).[12] Posaconazole was also associated with a lower rate of treatment failures compared with fluconazole/itraconazole (36% vs. 46%; P = 0.009). Adverse events were similar between the two study arms.[12]The other study involved 600 patients receiving allogeneic HSCT recipients with GVHD. Patients were randomized to receive posaconazole, 200 mg tid ( n= 301) or fluconazole, 400 mg qd ( n= 299) for up to 16 weeks.13 Although the incidence of total IFI during the 16-week study period was similar in posaconazole and fluconazole groups (5% vs. 9%; P= 0.07), the incidence of total breakthrough infections while on treatment was significantly lower in posaconazole-treated patients (2% vs. 8%; P= 0.003).[13] Aspergillus infections were significantly reduced among patients receiving posaconazole during the 16-week study period (2% vs. 7%; P= 0.0059) and as breakthrough infections while on treatment (1% vs. 6%; P= 0.001).[13] The overall mortality rate in posaconazole-treated patients was comparable with that of fluconazole arm (25% vs. 28%, respectively); however, IFI-related mortality was significantly lower in the posaconazole (1% vs. 4%) than fluconazole group (P =0.046).The side-effect profiles of the two agents were similar. [13]Based on these clinical trials, the NCCN[10], EBTM[11], ECIL[18], IDSA (the Infectious Diseases Society of America)[22] andBCSH ( British Committee for Standards in Hematology)[23] guidelines have defined a clear position for prophylactic use of posaconazole inimmunocompromised (AML/MDS with prolonged neutropenia) as well as post-HSCT with GVHD patients.
5. Summary and Conclusive Remarks
- In summary, considering the potential limitations and based on the pending cost utility analyses in our local setting, the “Shiraz IFI Study Group” yielded a relatively matching preference for antifungal agents as compared to international guidelines.[10, 11, 18, 22] The prophylactic indication of the available antifungal agents in high-risk patients were revisited and agreed upon. The algorithmic approach to the same is illustrated in Figure 2.Following data review and plenary discussions on local experiences, the “Shiraz IFI Study Group” panel admitted that the selection of antifungal agents for prophylaxis is a challenging and case-by-case decision. Although fluconazole retains a good activity against Candida species,[12,13] is not a mould-active azole thus is not shown to render protection against the life-threatening infections caused by Aspergillus, Zygomycetes and Fusarium species. Moreover, other than C.albicans, fluconazole is shown to provide modest activity against non-albicans species, i.e. C.glaberata and C.krusei. Therefore, as per guideline recommendations, high risk patients can be assigned to receive recommended antifungal prophylactic regimens as clinically deemed appropriate.
6. Disclosure
- The current report is a consensus summary from the “Shiraz IFI study Group” meeting on IFI prophylaxis in immunocompromised hosts, Dec 2012, Shiraz, Iran. This meeting has received organizational and scientific support from BehestanDarou PJS, Tehran, Iran. Authors declare no conflict of interest upon data review, talk delivery during the meeting and the preparation of this report. The co-authors M.A, L.N and MTN are affiliated to Behphar Scientific Committee, Behphar Group, Tehran, Iran.
ACKNOWLEDGEMENTS
- Authors would like to thank the Department of Internal Medicine, Shiraz University of Medical Sciences, Shiraz, as well as Dr. Malak Elsobky, Dr. Irina Alekseeva (MSD) and Dr. P. Dindoust, Dr. S.A. Hejazi Farahmand at Behphar Scientific Committee, Behphar Group, Tehran, for their scientific and organizational support to this meeting.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML