-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2012; 1(3): 21-32
doi:10.5923/j.ijim.20120103.03
Renal Disease as a Cardiovascular Risk Factor
Roberto Jorge da Silva Franco, Luis Cuadrado Martin
Department of Internal Medicine, Nephrology Section. Botucatu Medical School, Botucatu (SP), 18618-970, Brazi
Correspondence to: Roberto Jorge da Silva Franco, Department of Internal Medicine, Nephrology Section. Botucatu Medical School, Botucatu (SP), 18618-970, Brazi.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
An estimated 10% of the American population has some degree of renal disease, although asymptomatic. In Brazil, no data are available on the prevalence of chronic renal disease. A number of studies show a direct and close relationship between the degree of renal dysfunction and Cardiovascular Disease (CVD) risk. This increased CVD risk, despite being maximal in end-stage renal failure, begins to be noticed with slight declines in renal function. In addition, the presence of renal injury, even with normal renal function, evidenced by proteinuria or microalbuminuria, is also a potent CVD risk factor. At present, the primary causes of renal disease are diabetic nephropathy and hypertensive nephrosclerosis, accelerated by cigarette smoking and dyslipidemia. Hence, increased CVD risk in patients with chronic kidney disease may be secondary to the accumulation of these classical risk factors; however, frequency of CVD events in these patients is higher than that predicted by equations that take into account such classical factors. Therefore, there may be mechanisms intrinsic to renal lesion capable of accelerating systemic atherosclerosis. Accordingly, uremic toxicity itself, increased oxidative stress, change in the coagulation cascade, changes in lipid levels and hypervolemia are involved in the genesis of early atherosclerosis in patients with chronic kidney disease. It is incumbent on clinicians, nephrologists, hypertension specialists, and cardiologists to identify these patients through urinalysis, serum creatinine measurement, and microalbuminuria screening in order to reduce, through intensive treatment, to revert at least in part, the high CVD risk of that diseased portion of the population.
Keywords: Chronic Kidney Disease, Cardiovascular Disease, Renal Disease
Cite this paper: Roberto Jorge da Silva Franco, Luis Cuadrado Martin, Renal Disease as a Cardiovascular Risk Factor, International Journal of Internal Medicine, Vol. 1 No. 3, 2012, pp. 21-32. doi: 10.5923/j.ijim.20120103.03.
Article Outline
1. Historic Data
- In 1945, Langendorf and Pirani first described cardiac changes in post-mortem examinations performed on patients with chronic kidney disease (CKD). By gross analysis, these authors identified marked ventricular hypertrophy and, by microscopy, severe fibrosis and interstitial oedema. In their study, uraemia itself had been the cause of death, since routine dialysis was not available at that time[1].In 1975, Lindner et al. described a high prevalence of CVD disease with early atherosclerosis in the first longitudinal cohort study of patients in hemodialysis[2]. In 1960, in Seattle, 39 patients on average 37 years old by the time they started hemodialysis therapy, 23 (59%) died 6.5 years after joining the program almost 14 (61%) from CVD disease (CVD). CVD risk for this first cohort was estimated to be 30 times greater than that of the local general population. Thus, because of renal replacement therapy, life expectancy of patients with end-stage CKD increased, once the immediate cause of death from uraemia was eliminated, and CVD diseases became the main cause of death: at a younger age and at a much higher frequency than expected for the general population.
2. Glomerular Filtration and Proteinuria as a Cardiovascular Risk
- The decrease of glomerular filtration rate (GFR) and high proteinuria levels increase the risk of CVD. These associations have been shown in community-based populations (i.e., cohorts that were not selected specifically to enrol individuals with CKD or CVD), and in populations of patients at high CVD risk (i.e., cohorts in which patients with pre-existing CVD or CVD risk factors were specifically recruited).The best data come from a meta-analysis[3] of general population cohorts that included around 106 hundred thousand participants with urine albumin-to-creatinine ratio (ACR) measurements and near one million one hundred thousand participants with urine protein dipstick measurements where all had documented baseline estimated GFR[3]. Compared with participants who have estimated GFR normal (95 mL/min/1.73 m2), hazard ratios (HR) for all-cause mortality during 8 years of follow-up were approximately 1.2, 1.6, and 3.1 for estimated GFR of 60, 45, and 15 mL/min per 1.73 m2, respectively. Similar outcomes were observed for CV mortality, and either were obtained in older (> 65y) and younger (< 65y) individuals. Proteinuria was independently and multiplicatively associated with increased all-cause and CV mortality in this study. Compared to an albumin-to-creatinine ratio (ACR) of 6 mg/g, the adjusted HR for all-cause mortality was 1.2, 1.6 and 2.2 for ACRs of 10 mg/g, 30 mg/g and 300 mg/g, respectively, as near the same were observed for CV mortality. An ACR greater than 30 mg/g was associated with more than twofold mortality risk for all levels except the lowest (<30 mL/min per 1.73 m2). Estimated GFR (eGFR) less than 60 mL/min/1·73 m2 and ACR 10 mg/g or more are independent predictors of mortality risk in the general population. This study provides quantitative data for use of kidney’s measures, albuminuria and eGFR, for risk assessment and definition and staging of CKD.
3. The Burden of Chronic Kidney Disease
- CKD is recognized as a major global public health problem[4,5]. The disease affects 10–16% of the adult population in Asia, Australia, Europe, and the USA[6-9] and increases the risk of all-cause mortality, CVD, and progression to kidney failure, even after accounting for traditional risk factors such as hypertension and diabetes mellitus[4,10]. Therefore, even taking into account both under diagnosis and the predicted growth of the dialysis population in those all regions, most patients with renal dysfunction are expected to die before they require renal replacement therapy. In these patients, death is mainly due to CVD causes, and CVD risk for patients with CKD, although asymptomatic, is significantly higher than that predicted by the Framingham equations[11]. Framingham risk score, the traditional method to analyse future CV risk among individuals without CKD, provides poor overall accuracy in predicting CV events in patients with CKD[12]. This appears to be largely due to the markedly increased cardiac event rate and overall death rate among such patients. However altering the Framingham risk equations may increase their predictive accuracy. New prediction equations must be developed to help predict future events among patients with CKD. It should be emphasized that a patient with renal dysfunction is much more likely to die from CVD disease than from actual CKD.Data from the Hypertension Detection and Follow-up Program (HDFP) were the first to show a correlation between serum creatinine and CVD mortality among hypertensive patients. This association was independent of other cofactors evaluated: gender, race, blood pressure (BP), diabetes or degree of obesity[13]. Creatininemia thus emerged as a major predictor of CVD disease.The Hypertension Optimal Treatment (HOT) trial results have corroborated the relationship between creatininemia and CVD risk among hypertensive patients under drug therapy. In that study, serum creatinine was the most powerful risk predictor of the assessed factors[14]. Data from many other cohorts consistently point to renal dysfunction as an important CVD risk factor[15-20]
4. Pathogenecity of Chronic Kidney Disease
- The mechanisms by which CKD may lead to CVD disease are multiple. Traditional CV risk factors are defined as those in the Framingham Heart Study that have been used to estimate the risk of symptomatic ischemic heart disease[21]. Most of traditional CV risk factors, such as systolic hypertension, diabetes mellitus, older age, left ventricular hypertrophy (LVH), smoking, low HDL cholesterol are highly prevalent in CKD populations[10,22,23] and the number of CV risk factors appears to correlate with the severity of CKD[23]. The CV risk related by many traditional risk factors such as diabetes, older age and LVH largely parallels the relationship found in general population. Although some important differences have been noted with regard other risk factors. So, as an example, U-shaped relationships exist between mortality and BP and cholesterol levels in dialysis patients. The increased risk at lower levels of BP and cholesterol may reflect confounding from cardiomyopathy and malnutrition, although this has to be proved. The difference in CV prognosis in dialysis patients compared to those without renal disease is related in part to the clinical status of patients when they are started on dialysis[11, 24, 25, 27]. Based upon the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study, a large percentage of incident dialysis patients have traditional risk factors for CV disease[11]. This includes diabetes (54%), low serum HDL cholesterol (33%), hypertension (96%), LVH by ECG criteria (22%), and increased age, with the average patient age at the starting of dialysis program being nearly 60 years.Many dialysis patients have more than one of these risk factors, resulting in an even higher risk of adverse outcomes. As an example, among 373,539 United States dialysis patients, nearly 40 percent had a history of both diabetes and hypertension at the time of initiation of hemodialysis[27]. Patients who had both conditions had a five- to sixfold increased risk of having heart disease compared to those without history of either condition. Non-traditional risk factors that are relatively unique to patients with moderate to severe CKD alike play a role in the pathogenesis of CVD disease in patients with CKD. Non-traditional risk factors include variables routinely assessed in patients with CKD, such as: hyperparathyroidism and altered divalent ion metabolism[28] anaemia[29], and hydrosaline overload[30], uremic toxins, proteinuria, “increased inflammatory-poor nutrition” state[31,32] together with variables not routinely assessed in clinical practice: hyperhomocystinemia[33], increased oxidative stress, elevation levels of certain cytokines, endothelial dysfunction, changes in lipid levels not frequently assessed such as apolipoprotein(a)[34], build-up of asymmetric dimethyl arginine (ADMA)[35], inflammatory procoagulant activity[36], and abnormal behaviour of BP during sleep[37]. How these risk factors may lead to CVD is unclear. As an example, disorders of bone mineral metabolism in patients with CKD have often been linked with coronary artery calcification. However, while there is a consistent association between CKD and a higher burden of coronary artery calcification, the associations among phosphorus, calcium, and parathyroid hormone with coronary artery calcification in these patients is inconsistent[38,39]. Elevated levels of CRP and ADMA, both of which are typically found in patients with CKD, were both independently associated with an increased risk of all cause and CV mortality in the Modification of Diet in Renal Disease study (MDRD)[40,41], although a similar relation between CRP and CVD was not observed in the Irbesartan for Diabetic Nephropathy trial (IDNT)[42]. Hyperhomocysteinemia has also been inconsistently associated with increased CV risk[43].LVH is an independent CVD risk factor that, in the arterial hypertension setting, portends an ominous prognosis. A higher prevalence of LVH is found among patients with CKD, even in early stages, greater than would be expected for the degree of hypertension[30]. This prevalence becomes even higher as CKD progresses[44]. A number of mechanisms contribute to the pathogenesis of this disproportionate ventricular enlargement, beyond that expected for the degree of arterial hypertension, such as anaemia[45] and hyperparathyroidism[46].Our group showed the important role played by sodium retention, regardless of its hypertensive effect, in the development of this cardiac abnormality in patients with CKD[30]. Conversely, it should be noted that recent years have witnessed a sharp increase in chronic degenerative diseases (hypertension and diabetes mellitus) as cause of CKD, the same conditions that usually produce CVD lesion[24]. Therefore, by the time diabetes or hypertension renal lesion develops, a corresponding heart lesion usually already exists. Thus, a question rises as to whether mild renal dysfunction is only a marker of more extensive vascular damage or the very renal failure may be involved in the pathophysiology of CVD lesions.Some evidence points to a direct role of uraemia in the development of CVD disease. A study evaluating hemodialysis therapy during about 14 years in 16 young people, ages ranging from 7 to 30, demonstrated that 14 patients showed tomographic evidence of coronary artery calcification, compared to 3 out of 60 normal controls[47]. Moreover, some degree of coronary atherosclerosis was found in 80% of the cases of a series of autopsies performed on children who died during hemodialysis treatment[48]. This prevalence is clearly higher than that of coronary atherosclerosis in the general population of the same age.A second strong piece of epidemiological evidence supporting that uraemia "per se" is implied in the acceleration of atherosclerotic process comes from a series of patients who underwent coronary angiography. Among them, patients with increased creatinine levels were twice as likely to experience a coronary event. This heightened risk was independent of other comorbidities, including the angiographic pattern of coronary disease[20].
5. Importance and Methods to Detect Early Chronic Kidney Disease
- Detection of renal dysfunction is of utmost importance in clinical practice, for epidemiological studies in the United States, as already stated, suggest that approximately 20 million Americans have some degree of renal injury[5,24]. Additionally, 50% of cardiomyopathic patients show some degree of renal dysfunction, which often goesunder diagnosed[49]. As previously mentioned, renal dysfunction in patients with cardiomyopathy, coronary artery disease, and hypertension usually carries a worse prognosis. Patients with concomitant coronary insufficiency and renal artery atherosclerotic disease also have poor prognosis[50]. It has been postulated that this behaviour may be due to the activation of the renin-angiotensin-aldosterone system which invariably follows this clinical condition.The urine dipstick is a relatively insensitive marker for proteinuria, not becoming positive until protein excretion exceeds 300 to 500 mg/day (upper limit ≤150 mg, with most subjects being under 100 mg). Using a specific assay for albumin is a more sensitive technique. The normal rate of albumin excretion is <20 mg/day (15 µg/min); persistent albumin excretion between 30 and 300 mg/day (20 to 200 µg/min) is called microalbuminuria. Albumin excretion > 300 mg/day (200 µg/min) is considered to represent overt or dipstick positive proteinuria (also called macroalbuminuria). The confounding effect of variations in urine volume on the urine albumin concentration can be avoided by calculation of the urine ACR in an untimed urine specimen. A value 30 to 300 mg/g of creatinine (or, using standard (SI) units, 3.4 to 34 mg/mmol of creatinine) suggests that albumin excretion is between 30 and 300 mg/day and therefore that microalbuminuria is probably present[51]. Values above 300 mg/g (or 34 mg/mmol) are indicative of macroalbuminuria. This classification system requires that at least two of three specimens fall within the microalbuminuric or macroalbuminuric range[51]. This test has the following advantages: it does not require early morning or timed collections, it gives a quantitative result that correlates with the 24-hour urine values over a wide range of protein excretion, it is simple to perform and inexpensive and repeat values can be easily obtained to ascertain that microalbuminuria, if present, is persistent.It should be kept in mind that not only renal dysfunction, but also mere renal injury, identified by microalbuminuria, is associated with increased CVD risk, regardless of the presence of diabetes[52,53] or the severity of hypertension[54,55].In diabetics, the "non-dipper" pattern, that is, the lack of the physiological drop in nocturnal blood pressure[56], even among normotensive type I diabetics, is associated with microalbuminuria and often precedes its development. In cross-sectional studies[57,58] microalbuminuria is associated with target-organ damages, and in longitudinal studies represents an indicator of adverse prognosis regarding both general and CVD mortality[52-54]. Among over 9000 participants at high risk for a CV event in the HOPE trial, the presence of microalbuminuria was associated with an increased relative risk of the primary aggregate end point (myocardial infarction[MI], stroke, or CV death) in those with and without diabetes (1.97 and 1.61, respectively)[7]. The risk of an adverse CV event increased progressively with increasing absolute levels of microalbuminuriaPatients with microalbuminuria have an accumulation of associated risk factors, which may explain this excess mortality[58]; nonetheless, as with asymptomatic renal dysfunction, these patients' CVD risk is higher than that calculated by the predictive equations[59]. In addition to reflecting increased severity of target-organ damage, this excess CVD mortality is associated with endothelial dysfunction[60] and inflammatory changes[61], as well as changes in the coagulation and fibrinolytic systems[62]. Among non-diabetic patients with essential hypertension, those with microalbuminuria had higher plasma levels of von Willebrand factor (vWf) antigen than patients with normal albumin excretion[63]; furthermore, individually vWf and albumin excretion values were significantly correlated. vWf has been associated with occlusive thrombosis; thus, the increased plasma vWf levels might directly contribute to the enhanced CV risk. Low levels of microalbuminuria, well under the above definitions (≥30 mg/day[20 µg/min] or urine ACR ≥30 mg/g), are associated with an increase in CV risk that is additive to conventional risk factors. An increase in risk was also noted a post hoc analysis from the LIFE trial of patients with hypertension and ECG evidence of LVH. The urine ACR was measured in 7143 non-diabetic subjects (median value 10.2 mg/g creatinine) and 1063 subjects with diabetes (median value 26.9 mg/g creatinine)[64]. For every 10-fold increase in the ACR, the risk of the composite end-point of CV death, MI, or stroke increased by 57% and the risk of CV death by 98% among non-diabetics. The respective increases in risk for diabetics were 39% and 47%. A subsequent analysis of this trial showed that the risk of this composite end-point was reduced among participants who had a substantial reduction in microalbuminuria at the one-year follow-up[65].In the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study[66] 4447 patients with type2 diabetes was assigned to receive olmesartan (at a dose of 40 mg once daily) or placebo for a median of 3.2 years. Additional antihypertensive drugs, except angiotensin converting inhibitors (ACEI) or angiotensin II receptor blocker (ARB) were used as needed to lower BP to less than 130/80 mm Hg. The primary outcome was the time to the first onset of microalbuminuria. The times to the onset of renal and CV events were analysed as secondary end points. The target BP (<130/80 mm Hg) was achieved in nearly 80% of the patients. Microalbuminuria developed in 8.2% of the patients in the olmesartan group and 9.8% in the placebo group; the time to the onset of microalbuminuria was increased by 23% with olmesartan. A greater number in the olmesartan group than in the placebo group had fatal CV events — 15 patients (0.7%) as compared with 3 patients (0.1%) (P = 0.01), a difference that was attributable in part to a higher rate of death from CV causes in the olmesartan group than in the placebo group among patients with pre-existing coronary heart disease (11 of 564 patients[2.0%] vs. 1 of 540[0.2%], P = 0.02). Olmesartan was associated with a delayed onset of microalbuminuria, even though BP control in both groups was excellent according to current standards. The higher rate of fatal CV events with olmesartan among patients with pre-existing coronary heart disease is of concern.The Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA II) was the first trial to study[67] the class of AII antagonists in secondary prevention of nephropathy. The primary endpoint was to evaluate the role of irbesartan in the progression from incipient diabetic nephropathy, defined as the presence of microalbuminuria, to the next stage, at least 30 percent higher than the base-line level or to overt proteinúria. While 15% of the patients in the control group receiving conventional anti-hypertensive therapy, except ACEI and ARB, progressed to next stage, only 5% of the patients receiving the highest dose of irbesartan had their condition worsened. As the secondary endpoint, microalbuminuria was normalized (p=0,006), reverting, therefore, to normoalbuminuria in 34% patients treated with 300 mg irbesartan versus 21% in placebo group. As microalbuminuria is both CVD risk and marker of endothelial damage, the lower rate of cardiac events in the group treated with AII antagonists, due to decreased rates of abnormal urinary albumin excretion, stress the role of this anti-hypertensive class on target-organ protection. Regression of microalbuminuria may, therefore, be considered a goal to be achieved in the treatment of hypertensive patients.
6. Non-pharmacological Treatment of Chronic kidney Disease
- Non-pharmacological treatment is very important to control BP in CKD. The strictness of the diet depends on the degree of CKD. Na+ intake should be reduced to less than 60 mEq/day, and daily intake of proteins will depend upon renal function, but an average of 0.8–1.2 g//kg/day is recommended. Phosphorus intake is related to protein intake and must be less than 750 mg/day. Total caloric intake should never be less than 35 calories/kg/day, with carbohydrates around 50–60%, and saturated fats should be between 30–40% of total calories as long as plasma lipids are not elevated, in which case cholesterol should be reduced in the diet. Other dietary treatments are an increase in calcium intake, weight loss, moderate physical activity and tobacco/alcohol restriction[68].
7. Pharmacological Treatment of Chronic Kidney Disease
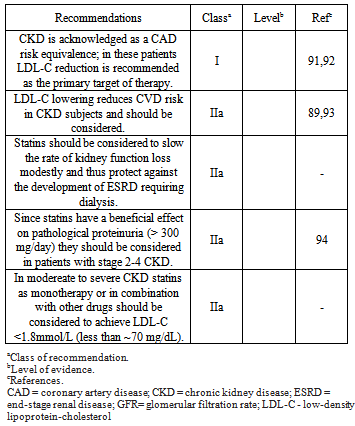
- As far as optimal BP is concerned, intensive BP control was found to be more likely to reduce CVD risk in the subgroup of patients with CKD than in the subgroup of patients with normal renal function, although this trend was not statistically significant[17]. Additionally, the analysis of interaction between anti-hypertensive therapy and renal function in the Systolic Hypertension Elderly Program (SHEP) trial revealed that treatment of isolated systolic BP was more effective in preventing CV events in older patients with CKD than in those with normal renal function[69]. Both in the HDFP[13] and in the MDRD[70] trial, renal protection was found to be enhanced in patients with more effective BP control. Thus, it seems that patients with CKD should be managed with tight BP control (130/80 mmHg), not only to provide renal protection, but also to optimize cardio protection.Many secondary analyses of studies that enrolled patients with known risk factors for CV disease, such as hypertension, diabetes, or any pre-existing CV disease, have shown that the presence or development of various degrees of CKD is independently associated with CV events[17,18,20,71-73]. The magnitude of this association was illustrated in meta-analysis of 10 cohorts with around 270 hundred thousand patients who had hypertension, diabetes, CVD, or a combination of these disorders[71]. Compared with a reference eGFR of 95 mL/min per 1.73 m2, the HR for CV mortality were 1.1 (for an eGFR of 60 mL/min per 1.73 m2), 1.7 (eGFR of 45 mL/min per 1.73 m2), and 3.1 (eGFR of 15 mL/min per 1.73 m2). Similar findings were noted for all-cause mortality, and the associations were similar in youth and senile patients. A monotonic association between higher ACR and risk of both CV and all-cause mortality was also noted. The current consensus[74] target for the treatment of hypertension is a BP of below 140/90 mmHg for all patients, and a BP of below 130/80 mmHg for those with diabetes or CKD. Recently added to the list of conditions warranting the lower BP target are coronary artery disease and coronary artery disease equivalents (stroke, carotid disease, aortic aneurysm, and peripheral vascular disease), as well as those individuals with a Framingham Risk Score of at least 10%. One theoretical issue with lower BP targets may be the existence of a J-shaped curve of BP versus CV event rate, implying a greater risk, especially of myocardial ischemia, of lowering diastolic BP, which is also the filling pressure of the coronary arteries, below the lower limit of coronary autoregulation. So, in hypertensive’s and borderline BP with high and very high CV risk or with 3 or more risk factors, diabetes, metabolic syndrome, target-organ damage and CKD with proteinuria > 1,0 g/l the target BP should be 130/80 mmHg.When a glomerular injury is present, especially with elevated proteinuria, ACEI and ARB are used most often, both in diabetic[75] and non-diabetic patients[76]. A new class of drugs[77,78], renin inhibitors (RI), has been introduced in the treatment of hypertension in CKD. They have a vasodilator effect on efferent arteriole reducing intraglomerular pressure and the mesangial fibrotic process. In severe CKD these drugs produce hyperkalemia, especially when they are associated with distal tubule diuretics or eplerenone. ACEI and RI doses should be reduced in advanced CKD (GFR < 15 ml/min), but this is not necessary with ARB. The three drugs have foetal toxicity and are contraindicated during pregnancy. Calcium antagonists have been recommended in CKD treatment due to their important antihypertensive and natriuretic effects. Dihydropiridines can cause vasodilatation of the afferent arteriole producing an increase in intraglomerular pressure [79]. Diltiazem and verapamil seem to provide greater kidney protection. Manidipine has demonstrated the greatest reduction of proteinuria due to its vasodilatory effect on both the afferent and efferent arterioles[80]. The most important side effects of calcium antagonists are local ankle oedema, headaches, flushing, tachycardia, and gingival hyperplasia. Diuretics are widely used medications in these types of patients since they are characterized by sodium and water retention[81]. When GFR is greater than 50 ml/min, thiazide diuretics alone, or in association with distal diuretics such as amiloride, triamterene, and spironolactone, can be administered. However, when GFR is less than 30 ml//min loop diuretics such as furosemide, bumetanide, ethacrynic acid, or torasemide should be administered, but not distal diuretics due to the possible increment of serum potassium. The most prominent side effects of diuretics are hypokalaemia, hyperuricaemia, dyslipidemia, glucose intolerance, insulin resistance, hyponatraemia, and hypomagnesaemia. Distal diuretics may cause hyperkalemia, skin rash, and gynaecomastia. Beta-blockers can be administered in order to counteract activation of sympathetic nervous system, but they can accumulate in advanced phases of renal failure. The non-selective beta-blockers should be carefully used in type 1 diabetic patients because they might inhibit hypoglycaemic signs and increase blood glucose levels[82]. Also in patients with severe peripheral vascular disease, they should aggravate the claudication or pain of peripheral artery occlusive disease. A significant side effect is bradycardia, especially in combination with other drugs like verapamil, diltiazem, and digoxin. Asthaenia, dyslipidemia, glucose intolerance, impotence, and hyperkalemia are other possible side effects. Alpha-blockers can be used not only for their vasodilator properties but also for their antiproliferative, platelet antiaggregant, and antiatherogenic effects. They are indicated in benign prostatic hypertrophy. The side effects are orthostatic hypotension, headache, mouth dryness, fatigue, and weakness. Combinations of two, three, or even more drugs are the rule in CKD, especially in diabetic patients. The most frequent combination is ACEI, ARB or RI with diuretics. If this is not sufficient, a calcium antagonist or a beta-blocker can be added. Combination therapy of ACEI and an ARB has been published with very good results, especially in patients with heavy proteinuria [83]. Combining an ACEI, ARB, or RI with a calcium antagonist has been recommended for a recent reappraisal of the European Society of Hypertension Guidelines[84]. ARB alone can be given in high doses[85].Recently it has been demonstrated that the addition of selective vitamin D receptor activation in patients with RAAS inhibition lowers the residual albuminuria and reduces renal risk in patients with diabetic nephropathy[86]. In many circumstances of CKD, an integrated treatment (antihypertensive, statin and anti-platelet therapy) should be considered. Available data from post-hoc analyses of statin trials provide evidence for the beneficial effects of statin therapy on CVD outcomes in patients with stages 2 and 3 CKD. The Pravastatin Pooling Project (PPP) included near 20,000 subjects with a median follow-up of 64 months[87]. The benefit was most marked in subjects with both CKD and diabetes. Notably there was also a 19% significant reduction in the risk of all-cause mortality. In the Heart Protection Study (HPS) the absolute risk reduction was 11% in a subgroup of subjects with mild CKD as compared with 5.4% in the total cohort[88]. The Study of Heart and Renal Protection[89] (SHARP) trial evaluated the efficacy of simvastatin plus ezetimibe compared with placebo in lowering cardiovascular morbidity in patients with CKD, approximately one-third of whom required maintenance dialysis SHARP reported results in 9500 high risk subjects with CKD. Major atherosclerotic events were reduced by 17% (P = 0.0022) and major vascular events by 15.3% (P = 0.0012) in patients on ezetimibe plus simvastatin as compared with placebo. SHARP included 3023 patients treated with maintenance dialysis. By design none had a history of myocardial infarction or coronary revascularization. During a median follow-up of 4.9 years, the combination of simvastatin and ezetimibe was associated with a trend toward benefit in lowering the incidence of the atherosclerotic CV events (15.0 versus 16.5 percent).CKD is acknowledged as a CAD risk equivalent. This has set the LDL-C reductions as the primary target of therapy. Non-HDL-C should be the second objective in the management of mixed dyslipidemia. The treatment algorithm should be based on GFR. Drugs eliminated mainly by the hepatic route should be preferred (fluvastatin, atorvastatin, pitavastatin, and ezetimibe). Statins metabolized via CYP3A4 may result in adverse effects due to drug–drug interactions, and special caution is required. The table 1 shows the recommendations for lipid for lipid lowering drugs in patients with moderate to severe CKD[90].Statins are generally well tolerated at moderate doses in subjects with CKD stages 1–2. Safety issues and dose adjustment become important in more advanced stages of CKD (stages 3–5), as adverse events are commonly dose related and due to increased blood concentration of the compound. Statins with minimal renal excretion should be the drug of choice (atorvastatin, fluvastatin, and pitavastatin).
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML