-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Internal Medicine
p-ISSN: 2326-1064 e-ISSN: 2326-1072
2012; 1(3): 11-16
doi:10.5923/j.ijim.20120103.01
Left Ventricular Hypertrophy and Its Correlates in Chronic Kidney Disease Patients in a Nigerian Tertiary Hospital
Daniel E Jesuorobo, James O Odia, Doris I Uchenna
Department of Internal Medicine University of Port Harcourt Teaching Hospital, Port-Harcourt. Nigeria
Correspondence to: Daniel E Jesuorobo, Department of Internal Medicine University of Port Harcourt Teaching Hospital, Port-Harcourt. Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Left ventricular hypertrophy (LVH) is an independent risk factor for mortality in patients with chronic kidney disease (CKD). This study aims to determine the prevalence of LVH in CKD patients as well as the risk factors for LVH in these patients, especially those with early CKD, using echocardiography. One hundred and fifty patients with CKD attending the medical clinics of a Nigerian tertiary hospital were studied. Seventy-five healthy controls, matched for age sex and body mass index were also recruited. Clinical and laboratory parameters as well as echocardiographic indices were measured. LVH was present in 76% of the patients and 6.7% of the controls. Anemia was found in 81.3% of patients and was most severe in patients with end-stage kidney disease of which 87% had anemia. Mean arterial pressure and eGFR were identified as predictors of LVH among the patients with SBP being the sole predictor of LVH in patients with CKD stage 3. This study shows a high prevalence of LVH in CKD patients and a strong relationship between CKD and LVH. The isolation of SBP as the sole predictor of LVH in patients in CKD stage 3 underscores the need for aggressive control of hypertension in these patients.
Keywords: Chronic Kidney Disease, Left Ventricular Hypertrophy, Nigerian
Cite this paper: Daniel E Jesuorobo, James O Odia, Doris I Uchenna, Left Ventricular Hypertrophy and Its Correlates in Chronic Kidney Disease Patients in a Nigerian Tertiary Hospital, International Journal of Internal Medicine, Vol. 1 No. 3, 2012, pp. 11-16. doi: 10.5923/j.ijim.20120103.01.
Article Outline
1. Introduction
- Chronic kidney disease is a major cause of cardiovascular morbidity and mortality worldwide and accounts for a significant proportion of deaths in hospitals across Nigeria and most parts of Africa.[1] In developed countries like the United States of America, cardiovascular disease is frequently associated with chronic kidney disease (CKD) and individuals with CKD are more likely to die of cardiovascular disease than kidney failure.[2] Among dialysis patients, cardiac disease was found to be the major cause of death accounting for 40% of deaths in international registries.[3] Many of the cardiac deaths in patients with CKD have been related to a high prevalence of left ventricular hypertrophy (LVH) though confounding factors of LVH seen in these patients include hemodynamic factors such as hypertension, fluid overload and anemia and non hemodynamic factors such as metabolic and endocrine abnormalities, autonomic dysfunction, ischemic heart disease and cardiomyopathy.[4] These factors cause volume and/or pressure overload and thought to be the primary cause of LVH.[5] Left ventricular hypertrophy is an early subclinical marker of cardiovascular disease and heart failure risk.[6] In patients with end-stage renal disease, LVH is common and is an independent predictor of cardiovascular disease and mortality[7] and it is suggested that it might already be present in a significant proportion of patients with CKD in the early stages.[8] This study aims to determine the prevalence of left ventricular hypertrophy and its correlates in CKD patients being managed in a tertiary hospital in Port Harcourt, Nigeria. It also tries to establish the presence of LVH in the earlier stages of CKD which could explain the high mortality among this group of patients.
2. Subjects and Methods
- One hundred and fifty patients and 75 apparently healthy age and sex matched controls who gave informed consent, were recruited for the study over a 12 month period at the medical outpatient clinic of the University of Port Harcourt Teaching Hospital after obtaining ethical approval from the ethical committee of the hospital. Exclusion criteria included patients receiving dialysis treatment, patients with arterio-venous fistulae, history of cigarette smoking and those less than 18 years. Demographic information and disease related variables were obtained using well structured questionnaire interview. Blood pressure was measured using Accoson’s mercury sphygmomanometer to determine the brachial artery systolic and diastolic blood pressures using the first and fifth Korotkoff sounds respectively. The blood pressure was taken in the supine and erect positions. Exercise, smoking and caffeine were avoided at least 30 minutes prior to blood pressure measurement. Hypertension was defined as mean arterial pressure ≥ 105mm Hg and/or SBP ≥ 140 mmHg and/or DBP using fifth korotkoff phase ≥ 90 mm Hg.[9] Hypertension was classified according to JNC VII:7 Normal: SBP <120 mmHg and DBP <80 mmHg Pre-hypertension: SBP 120-139 mmHg or DBP 80-89 mmHg. Hypertension stage 1: SBP 140-159 mmHg or DBP 90-99 mm Hg. Hypertension stage 2: SBP >160 mm Hg or DBP >100 mm Hg.When a person’s average systolic and diastolic pressures fall into different stages, the higher stage applies.7Height in meters was measured using a stadi-o-meter with subject standing feet together without shoes or head gear, back and heel together against a vertical ruled bar to which a movable attached horizontal bar is brought to the vertex of the patient’s head and reading taken to the nearest 0.5 centimeter. Weight was taken using a weighing scale with the subject wearing only light clothing. The scale used was standardized against fixed weight at every ten readings. Body mass index- weight in kilograms divided by the square of the height in meters (kg/m2) was calculated. The body surface area (BSA) was also calculated using the formula BSA= (.0001)(71.84) (Wt.425) (Ht.725), with weight in kilograms and height in centimeters.[10] Waist and hip circumference was measured in centimeters (cm) using a tape measure. Waist circumference was taken by the author at the part of the trunk located midway between the costal margin and the iliac crest while the subject stands with feet about 25- 30 cm apart and measured to the nearest 0.5 cm at the end of normal expiration.[11] Hip circumference was taken at the level of the greater trochanters. The waist hip ratio was also calculated. A venous blood sample of about 7 mls was drawn from subjects for the determination of hemoglobin, creatinine levels and fasting lipid profile. Creatinine clearance was calculated using the Cockroft and Gault equation,[12] which has been shown to correlate well with measured creatinine clearance in Nigerians.[13]
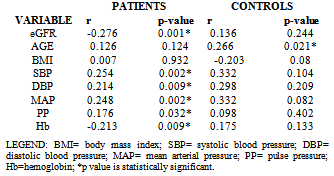
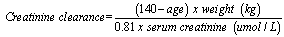 For females multiply by factor of 0.85Degree of renal impairment was classified using the Kidney Disease Quality Outcome Initiative (K/DQOI) classification:1Stage 0: GFR ≥90 mL/min with no renal damageStage 1: GFR ≥90 mL/min with renal damageStage 2: GFR 60 – 89.9 mL/minStage 3: GFR 30 – 59.9 mL/minStage 4: GFR 15 – 29.9 mL/minStage 5: GFR <15 ml/min.Patients were organized into three groups based on their e-GFR with 50 patients each in stages 3, 4 and 5 of CKD. Thereafter all recruited patients were evaluated using echocardiography to determine left ventricular hypertrophy. The control group was similarly evaluated.Blood samples were taken from the patients and serum creatinine, hemoglobin and fasting lipid profile ( total cholesterol, triglyceride, HDL and LDL) estimations were carried out on all the patients. Two dimensional motion mode guided echocardiograms were performed by the author on all patients and controls with transthoracic echocardiography using ALOKA 400 ultrasound imaging system. The echocardiography view that was utilized for the study is the parasternal long axis view with the patient in left lateral decubitus position. Left ventricular mass was calculated using the American society of echocardiography formula modified by Devereux.[14]LV mass (g)= 0.8(1.04(IVSd + LVIDd + PWTd)3 + 0.6 whereIVSd= interventricular septal thickness in diastolePWTd= posterior wall thickness in diastoleLVIDd= left ventricular internal diameter in diastoleLeft ventricular mass indexed to body surface area was determined by M-mode and 2- dimensional echocardiography. Left ventricular hypertrophy was defined in absolute terms as LVMI >134 g/m2 in men and >110 g/m2 in women.[15]Statistical Analysis: For continuous variables, mean values and standard deviations were calculated and the means compared by using the independent sample t test. Categorical variables were compared using the chi-square test. The cross tabulation was used to analyze the relationship between the stages of CKD and LVH and other variables. Pearson’s correlation was used to assess the relationship between LVMI and the variables (age, sex, body mass index, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, pulse pressure, hemoglobin, e-GFR) affecting it. Variables with significant correlation were further analyzed using the standard method of linear regression analysis to isolate possible determinants of LVMI based on their level of importance. P<0.05 was taken to be statistically significant.
For females multiply by factor of 0.85Degree of renal impairment was classified using the Kidney Disease Quality Outcome Initiative (K/DQOI) classification:1Stage 0: GFR ≥90 mL/min with no renal damageStage 1: GFR ≥90 mL/min with renal damageStage 2: GFR 60 – 89.9 mL/minStage 3: GFR 30 – 59.9 mL/minStage 4: GFR 15 – 29.9 mL/minStage 5: GFR <15 ml/min.Patients were organized into three groups based on their e-GFR with 50 patients each in stages 3, 4 and 5 of CKD. Thereafter all recruited patients were evaluated using echocardiography to determine left ventricular hypertrophy. The control group was similarly evaluated.Blood samples were taken from the patients and serum creatinine, hemoglobin and fasting lipid profile ( total cholesterol, triglyceride, HDL and LDL) estimations were carried out on all the patients. Two dimensional motion mode guided echocardiograms were performed by the author on all patients and controls with transthoracic echocardiography using ALOKA 400 ultrasound imaging system. The echocardiography view that was utilized for the study is the parasternal long axis view with the patient in left lateral decubitus position. Left ventricular mass was calculated using the American society of echocardiography formula modified by Devereux.[14]LV mass (g)= 0.8(1.04(IVSd + LVIDd + PWTd)3 + 0.6 whereIVSd= interventricular septal thickness in diastolePWTd= posterior wall thickness in diastoleLVIDd= left ventricular internal diameter in diastoleLeft ventricular mass indexed to body surface area was determined by M-mode and 2- dimensional echocardiography. Left ventricular hypertrophy was defined in absolute terms as LVMI >134 g/m2 in men and >110 g/m2 in women.[15]Statistical Analysis: For continuous variables, mean values and standard deviations were calculated and the means compared by using the independent sample t test. Categorical variables were compared using the chi-square test. The cross tabulation was used to analyze the relationship between the stages of CKD and LVH and other variables. Pearson’s correlation was used to assess the relationship between LVMI and the variables (age, sex, body mass index, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, pulse pressure, hemoglobin, e-GFR) affecting it. Variables with significant correlation were further analyzed using the standard method of linear regression analysis to isolate possible determinants of LVMI based on their level of importance. P<0.05 was taken to be statistically significant. 3. Results
3.1. Demographic Characteristics of the Population
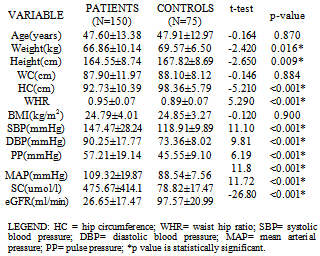
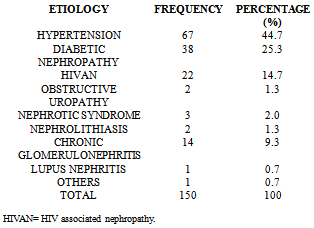
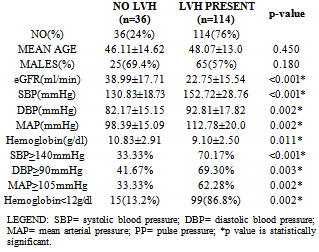
- Table 1 shows the socio-demographic characteristics of the study population. Of the 150 patients, 90 (60%) were males and 60(40%) were females with a male:female ratio of 3:2. The controls consisted of 43(57.3%) males and 32(42.7%) females. The age of the study population ranged from 19 to 65 years with a mean age of 47.6±13.4 years. The mean body mass index of both patients and controls were 24.8±4.0 and 24.9±3.3 kg/m2 respectively with no significant difference statistically between them. The prevalence of hypertension in the study population was 70.7% and was as high as 86% among patients with CKD stage 5. The etiology of CKD among the patients is illustrated in table 2 which shows hypertension (44.7%) as the commonest cause of CKD followed by diabetic nephropathy (25.3%) and HIVAN (14.7%).
|
|
|
3.2. Prevalence of LVH
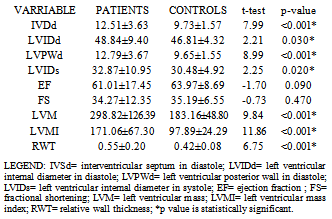
- One hundred and fourteen patients had LVH as determined by left ventricular mass index giving a prevalence of 76%. Among the patients, 65(72.2%) men and 49(81.7%) women had LVH with no significant difference statistically between the proportion of men and women with LVH. Only 5(6.7%) of the controls had LVH and the mean LVMI among them was 97.9±24.3g/m2. The other left ventricular chamber dimensions and indices of systolic function in patients and controls are shown in table 4.
|
|
3.3. Anemia in CKD
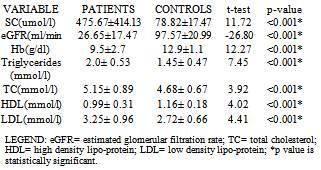
- One hundred and twenty-two (81.3%) patients had anemia compared with only 12 (16%) controls. The mean hemoglobin was significantly lower in patients (9.5±2.7g/dl) than controls (12.9±1.1g/dl). (t=12.27, p=<0.001). More patients in stage 5 CKD had anemia (87%) when compared with patients in stage 3 CKD (52.6%) and the degree of anemia was more severe in patients with endstage renal disease. (12.9±1.1g/dl vs 7.33±1.75g/dl).
3.4. Dyslipidemia in CKD
|
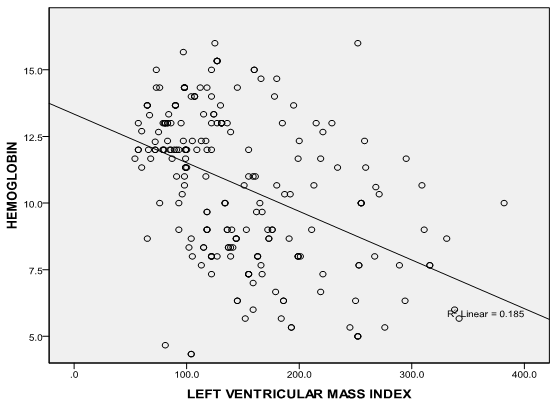 | Figure 1. Correlation graph showing the relationship between serum hemoglobin and LVMI |
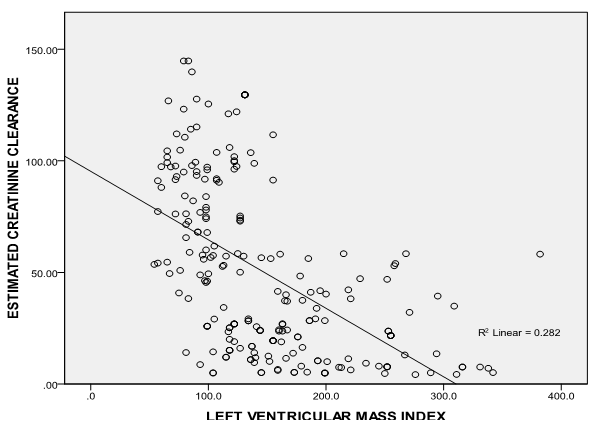 | Figure 2. Correlation graph showing the relationship between LVMI and eGFR |
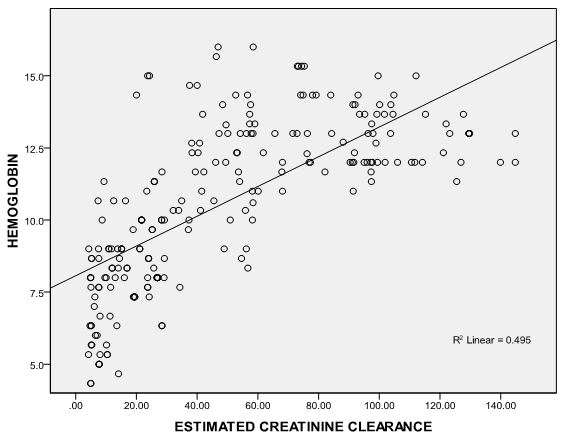 | Figure 3. Correlation graph showing the relationship between serum hemoglobin and eGFR |
4. Discussion
- It is well established that patients with kidney failure are at highest risk of mortality from cardiovascular disease (CVD).[16] The United States National Kidney Foundation (NKF) Task force on cardiovascular disease (CVD) recommends that patients with CKD be placed in the highest risk group for subsequent CVD and identified LVH as a main target of intervention.[17]The prevalence of LVH among patients in this study was found to be 76% and this finding clearly shows the high prevalence of LVH in predialysis CKD patients and is similar to finding from other studies which have shown prevalence between 40- 80% before initiation of renal replacement therapy.[18][19] It is noteworthy that LVH prevalence in this study population, though not as high as that reported by Ulasi et al,[20] is much higher than that reported by Agaba et al.[21] The low prevalence of LVH (14%) reported by Agaba et al12 may be because of his study population who were exclusively diabetic patients. This study demonstrated that LVH was already high in CKD stage 3 (56%) and increased progressively along with the decline in eGFR, attaining the greatest prevalence in patients with eGFR <15ml/min (90%). Paoletti et al6 and Levin et al[22]also documented a similar trend and this clearly suggest that in patients with CKD, LVH occurs earlier than expected and is already present well before the onset of overt uremia.Most patients in the study population had hypertension (70.7%) and it was also found to increase with declining eGFR to as much as 86% in CKD stage 5. This is not surprising as it is one of the factors shown to best predict LVH. In fact, the prevalence of hypertension in patients with CKD is very high, accounting for approximately 83% in the cohort of the Modification of Diet in Renal Disease Study(MDRDS).[23] A direct association was observed between LVMI and SBP, DBP, PP and MAP consistent with previously reported data from dialysis patients.[24] Of the variables tested, only MAP and eGFR predicted the occurrence of LVH. This is similar to findings from other studies in which LVH was more closely associated with MAP and pulse pressure.6,11 The level of blood pressure is often lower than one might expect to account for the dramatic increase in the prevalence of LVH. The mean differences in the level of systolic blood pressure that predict severity of LVH are small (approximately 20 mmHg in this study), but consistent with other reports in the literature.[25][26] This study demonstrates a statistically significant difference in systolic blood pressure between those with and without LVH. The multivariate analysis results are concordant with those of other researchers[27] in that hypertension appears to be related to left ventricular hypertrophy, but it does not explain a large amount of the variability in LVH.The hemoglobin levels of the study population had a negative correlation with LVMI which was statistically significant. This finding is similar to a previous report by Ulasi et al11 in which a strong relationship was found between anemia and the prevalence of LVH in patients with chronic renal failure. Anemia has been consistently associated with cardiovascular morbidity and LVH in the ESRD population and the results of this study are consistent with those in the literature.[28][29] Anemia leads to an increase in cardiac workload due to relative tissue ischemia, which subsequently leads to development of LVH.10 Several studies have been done to demonstrate if the reversal of anemia, using erythropoietin therapy, results in partial regression of LVH in the dialysis population with conflicting results.[30][31] This study presents an opportunity to study the impact of correction of anemia on LVH in the pre-dialysis population.Estimated glomerular filtration rate correlated negatively with left ventricular mass index and also emerged the strongest predictor of LVMI in patients with CKD accounting for 24.1% of the variation in LVMI. Other variables like total cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol had significantly higher mean values in patients compared with controls but none had any correlation with LVMI. Of the risk factors for LVH identified for patients with early CKD, only systolic blood pressure predicted LVH. Systolic blood pressure was found to account for 30.8% of the variation in LVMI in patients with early CKD thus demonstrating the importance of SBP control in the management of these patients. The main limitation of our study is that it is a single center study and the long term outcome for our patients is not available.
5. Conclusions
- In conclusion, this study shows that LVH occurs early in CKD in Nigerian patients and increased dramatically with declining renal function. It also established SBP as the only risk factor that predicted LVMI in patients with early CKD hence making a case for aggressive control of hypertension, especially systolic blood pressure to prevent development of LVH with its attendant complications. There are numerous problems facing the health sector in Nigeria such as poor health infrastructure and shortage of well trained health personnel. The poor health seeking habits of patients with chronic kidney disease in Nigeria also contribute to the poor outcomes. Many seek alternative treatment and only come to the hospital when complications have set in. There is need for mass enlightenment campaigns to educate the populace on prevention of chronic kidney disease with its associated problems.
Transparency Declaration
- None to declare.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML