-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Hydraulic Engineering
2012; 1(5): 48-54
doi: 10.5923/j.ijhe.20120105.04
Biofilm Formation by Legionella pneumophila in Water Distribution Systems: Role of Supports and Temperatures
Jalila Tai 1, 2, Mostafa Mliji 1, Mohamed Nabil Benchekroun 2, Mly Mustapha Ennaji 3, Mariam Mekkour 1, 4, Hayat Ennaji 1, Nozha Cohen 1
1Division de Microbiologie et d’Hygiène des Produits et de l’Environnement, Institut Pasteur du Maroc, Casablanca, 20360, Maroc
2Laboratoire de Biotechnologie, de l’Environnement et de la Santé, Faculté des Sciences et Techniques, Université Hassan II-Mohammedia, 146, Maroc
3Laboratoire de Virologie, Hygiène et Microbiologie, Faculté des Sciences et Techniques Université Hassan II-Mohammedia, 146, Maroc
4Laboratoire Diversité et Conservation des Systèmes Biologiques, Faculté des Sciences, Université Abdelmalek Essaadi-Tétouan, 93 000, Maroc
Correspondence to: Nozha Cohen , Division de Microbiologie et d’Hygiène des Produits et de l’Environnement, Institut Pasteur du Maroc, Casablanca, 20360, Maroc.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Legionella pneumophila, the aetiological agent of 90% of legionellosis cases, is a common inhabitant of natural and in man-made aquatic environments, where it’s able to reside in biofilms. These biofilms represent a sophisticated network of metabolic and architectural interactions, which concentrates nutrients and protects the microbial residents from physical, chemical and biological hazards. L. pneumophila can form biofilms, where they become integrated and survive for days to weeks in water distribution systems depending of the environmental conditions. The materials of water distribution systems varied in their abilities to support biofilm development and the growth of L. pneumophila. Our aims of this study are to determine the kinetic of ability two strains serogroups L. pneumophila to adhere and form biofilm on three different surfaces (stainless galvanized, Copper and polyethylene) commonly used in hot water distribution system in Morocco at three growth temperatures 20, 37 and 44℃. L. pneumophila serogroup 2-15 revealed high capability to adhere and form biofilm on the stainless steel and polyethylene than serogroup 1 in 37℃ assayed incubation temperature than at 20 and 44℃. In contrast, copper was found may be to inhibit both biofilm growth and the colonization of water systems by L. pneumophila at all temperatures tested. In conclure, the selection of the suitable pipe material capable minimize the possibility of biofilm development associated in hot water distribution systems and reduce Legionnaires’ disease.
Keywords: Legionella Pneumophila, Biofilm, Temperature, Materials Surface
Cite this paper: Jalila Tai , Mostafa Mliji , Mohamed Nabil Benchekroun , Mly Mustapha Ennaji , Mariam Mekkour , Hayat Ennaji , Nozha Cohen , "Biofilm Formation by Legionella pneumophila in Water Distribution Systems: Role of Supports and Temperatures", International Journal of Hydraulic Engineering, Vol. 1 No. 5, 2012, pp. 48-54. doi: 10.5923/j.ijhe.20120105.04.
Article Outline
1. Introduction
- In drinking water distribution systems, all surfaces in contact with water can be colonized bymicroorganisms[1-2]. One of the most common ways for bacteria to live is adhering onto surfaces and forming organized communities named biofilms[3],[4-5]. Biofilms are mostly known on solid surfaces, although they occur in a vast range of manifestations[6]. Drinking water biofilms are formed predominantly by microorganisms of the autochthonous aquatic microflora without any relevance to human health[7]. However, drinking water biofilm has the potential to harbor opportunistic pathogens which can harm human health, especially in immunocompromised people[8-9]. Once integrated in a drinking water biofilm, these organisms are protected from external stresses such as the action of disinfectants and can thus persist and possibly multiply inside the biofilm[7]. Contamination of drinking water occurs when opportunistic pathogens are released from a biofilm as a consequence of physical disturbance or active detachment of infectious cells, which then pose a potential threat to human health[8-10]. This microbial colonization may be even more relevant in hot water leaving from building plumbing systems, where opportunistic pathogens such as Legionella pneumophila find optimal growth conditions[11]. Multiplication of Legionella in hot water systems poses a potential health threat when water use leads to aerosol formation[12]. Bacteria of the family Legionellaceae are Gram-negative bacilli that belong to the gamma-2 subdivision of the Proteobacteria and comprise Legionella as sole genus[13-14]. Currently, the Legionella genus includes 54 species[15-16] and more than 70 different serogroups, and more than 23 species have been proven to be causative agents of Legionnaires’ disease[17]. L. pneumophila is the major cause of outbreaks (91.5%) and L. pneumophila serogroup (sg) 1 has been recognized as the most important agent in this regard (84.2%)[18].Legionella can survive in varied water conditions, in temperatures of 0 - 68℃, a pH range of 5.0 - 8.5[19], and a dissolved oxygen concentration in water of 0.2 - 15 ppm[20]. With the exception of natural hot springs where temperature ranges from 35℃ to 40℃, the sources of legionellosis are exclusively man-made water systems[21]. In water, a temperature range between 20℃ to 45℃ favors the growth of L. pneumophila[21]. Legionella are able to enter and to develop biofilms in hot water systems. While bacteria occupy niches suitable for their survival and growth which function as amplifiers or disseminators of these microorganismes. Human exposure to Legionella-contaminated sources can result in outbreaks of legionellosis.The aim of this study was to determine and evaluate the kinetic of ability L. pneumophila to adhere and biofilm formation on three different surfaces commonly used in hot water system in Morocco at three growth temperatures.
2. Materials and Methods
2.1. Bacterial strains and Culture Conditions
- Legionella pneumophila sg 1 and Legionella pneumophila sg 2-15 isolates from water systems were grown without agitation in filter-sterilized BYE (5 g ACES/L, 10 g yeast extract /L; pH 6.9) supplemented with L-cysteine (0.4 g/L) and ferric pyrophosphate (0.25 g/L). Solid medium was obtained by adding charcoal (2 g l/L) and agar (15 g/L) to non-sterilized BYE. The resulting medium (BCYE-agar) was autoclaved and then supplemented with L-cysteine and ferric pyrophosphate prior to use and the pH was adjusted to 6.9 with 1 M potassium hydroxide. Colonies growing on BCYE agar were definitively identified as Legionella spp. using a commercially available latex agglutination test (Slidex Legionella- kit, BioMérieux) that distinguishes Legionella pneumophila serogroup 1, Legionella pneumophila serogroups 2 to 15 (polyvalent), and Legionella anisa.
2.2. Kinetics of Biofilm Formation
- L. pneumophila biofilm formation was monitored using polystyrene 96-well microtitre plates (Nunclon MicroWell Plates, Nunc). Three distinct procedures were used in this study. (1) Biofilm formation at different temperatures was assayed using cells grown on BCYE plates at 37℃, 5% CO2 for 3–4 days. Cells were resuspended in BYE at OD600 0.2 and then aliquoted (150 mL) into ten wells of a 96-well microtitre plate, which was incubated at 20, 37 or 44℃ in 2, 6, 9 and 12 days. Medium and non-adherent cells were then removed by aspiration and the wells were rinsed with BYE (200 mL). Cells that had adhered to the wells were stained with 200 mL 0.3% crystal violet at room temperature for 15 min. Excess crystal violet was then removed and the wells were washed three times with water (400 mL). Fixed crystal violet was solubilized in 200 mL 100% ethanol, 15 min at room temperature. Biofilm formation was estimated by measuring the A550 of each well using a microtitre plate reader and calculating the mean from the ten wells. (2) Assays for adhesion were performed using cells grown at 20, 37 and 44℃ in BYE liquid cultures, harvested at different growth phases (48, 120 and 192 h of growth for exponential, stationary and late stationary phase, respectively) and resuspended in BYE at OD600 2.0. Cell suspensions were then aliquoted (150 mL) into ten wells of a 96-well microtitre plate, which was incubated at 20, 37 and 44℃ for 6 h. Adhesion was estimated by crystal violet staining of adherent cells, as described above. (3) Assays for biofilm growth were done in a similar way using cells grown in BYE at 20, 37 and 44℃ until late stationary phase. After 6 h incubation at 20, 37 and 44℃, medium and non-adherent cells were removed and 150 mL BYE were added to the wells. Incubation was continued for 7–14 days. Where indicated, medium and nonadherent cells were removed and replaced by sterile BYE on day 7. After incubation, biofilm formation was quantified by crystal violet staining, as described above.
2.3. Biofilm Formation
2.3.1. Surfaces and Cleaning Treatment
- Three different surfaces used for biofilm experiments were employed: stainless galvanized, Copper (Cu) and polyethylene (PE). Sections of each of the plumbing material tubes were cut open to make coupons. The tube was cut into sections with an inside area of 1 cm2, and a 1 mm diameter hole was drilled so that the chips could be suspended from titanium wires. Materials that are commonly used in the construction of water systems were selected. The materials were all commercially available and were obtained from a local plumbing stockist.Before each experiment, the test materials were cleaned in the form of chips (1 cm2) : polyethylene, which has been treated with wash in sterile demineralized water for 15 minutes, soaking in a chlorine solution to 150 mg / L (chloine in sterile water) for 15 min, and washing with sterile deionizer water for 4 times. The chips (stainless galvanized, cooper ) which were washed in sterile demineralized water for 15 minutes and soaked with 95% ethanol for 10 min, and rinsed six times for 5 min each with demineralized water, and then the surfaces were autoclaved for 15 min at 120°C. Finally, the surfaces are oven drying at 50°C overnight in a petri dish in clean conditions.
2.3.2. Adhesion of L. pneumophila
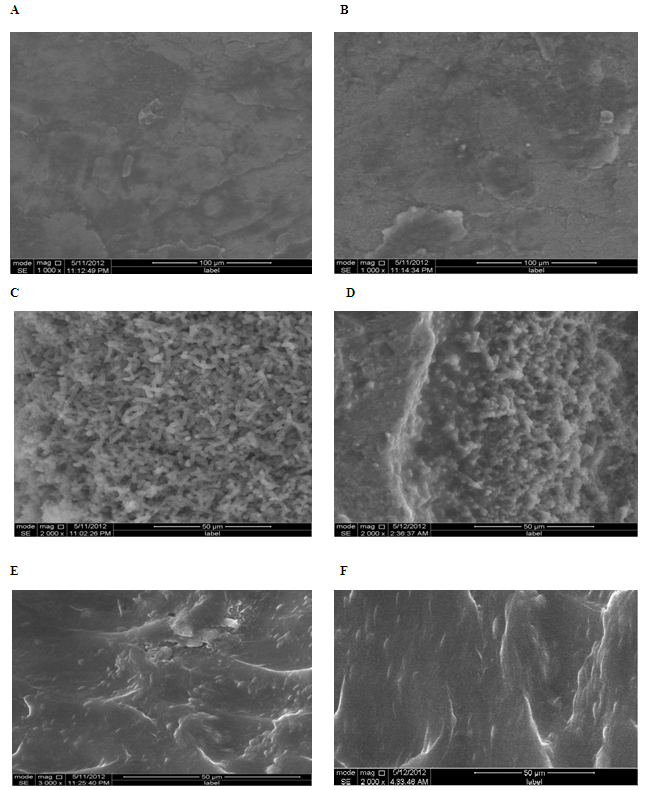
- Cells (in the late stationary phase of growth) were resuspended in BYE at OD600 2. Seven milliliters of the bacterial suspension was poured into a petri dish (55-mm diameter) containing a chip of surface material and stored at 20, 37, or 44℃. The medium was replaced after 6 h and then every 24 h for studies at 20, 37 or 44℃. Cell adhesion and biofilm formation were evaluated at each temperature by Environmental Scanning Electron Microscopy cell of the contaminated surface after 14 days.
2.4. Environmental Scanning Electron Microscopy
- For Environmental scanning electron microscopy (model: FEI Quanta 200 equipped with EDAX sensor for microanalysis of surfaces), the tiles of plumbing materials were removed from the culture bacteria were removed by gentle rinsing in sterile water.
3. Resultat
- The ability of L. pneumophila strain to form a monospecies biofilm was investigated at different growth temperatures and incubation days in order to determine the most favorable conditions for biofilm establishment.Microtitre plates were inoculated with L. pneumophila serogroup 1 and L. pneumophila serogroup 2-15 and incubated at 20, 37 or 44℃ for 3, 6, 9, and 12 days for each temperature. As the procedure originally described by O’Toole and Kolter[22] yielded only low maximum values for L. pneumophila (A550<1.0), we optimized the crystal violet stain assay as described in Materials and Methods. Culture growth was monitored as OD600 and the number of sessile cells within the obtained biofilms was estimated by measurement of A550 after crystal violet staining of the biomass attached to the surfaces of 96-well plates. The data in (Fig. 1) show the growth kinetics of ability of L. pneumophila to adhere at 20, 37 and 44℃. In the above data, the quantity of the biofilms formed by L. pneumophila strains (Fig. 1: strains sg 1 and 2-15) was observed to be influenced by temperature. Biofilms were generally formed more extensively at temperatures at 20℃ and 37℃ than at 44℃. The temperature dependence could also be observed in the speed of formation and the adherence stability of the biofilms of L. pneumophila strains.It clearly appeared that biofilm formation by L. pneumophila strain was favored at 37℃ for 6 days for incubation since A550 as compared to 20 and 44℃. The result showed also that at 20, 37℃ and 44℃ L. pneumophila sg 2-15 has distinctly greater biofilm-forming capacity than other L. pneumophila sg 1. All L. pneumophila strains revealed that similar trends biofilms were formed faster at 3 to 6 days period of observation at 20, 37 and 44℃, while biofilms were formed slowly 9 and 12 days. We also observed that biofilms formed at 37℃ were more stably attached than those formed at 20 and 44℃, which became detached during the washing steps prior to crystal violet staining.In order to optimize biofilm formation, we tested the influence of growth phase on adhesion of L. pneumophila sg 1 and sg 2-15. Bacteria were grown at 20, 37 and 44℃ of cells from exponential, stationary and late stationary phase were used to inoculate 96-well microtitre plates. After 6 h of incubation at 20, 37 and 44℃, the number of adhered cells was estimated by crystal violet staining (Fig. 2). This clearly showed that the adhesion ability increases during growth and that, consequently, cells in late stationary phase exhibit the highest capacity for adhesion.
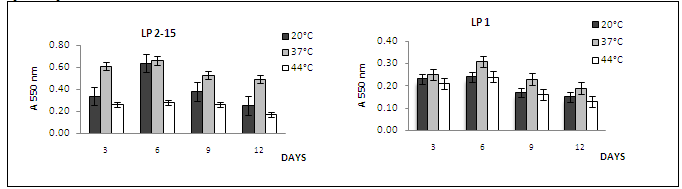 | Figure 1. Growth kinetics of ability of L. pneumophila serogroups to adhere at of at 20, 37 and 44℃ according to the day numbers |
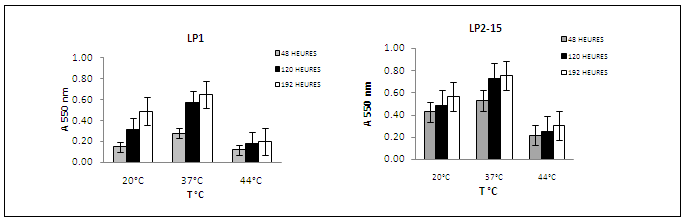 | Figure 2. Adherence of L. pneumophila serogroups at 20, 37 and 44℃ according to growth phase |
 | Figure 3. Comparison between two strains of L. pneumophila serogroups biofilm formation after 14 days at 20, 37 and 44℃ |
4. Discussion
- All bacteria produce multiple adhesins permitting organisms to switch from planktonic to sessile forms (or the other way around) triggered by different environmental conditions[23]. However, the success of bacterial attachment to an available surface is dictated by a number of variables, including environmental factors (e.g.,growth temperature) and various properties of the cell surface[24-25]. We report here the development of a monospecies biofilm for L. pneumophila, a bacterium known to form biofilms in nature. Although these results do not reflect the complexity found in natural environmental biofilms, monospecies biofilms were the first step towards understanding adaptation to the sessile lifestyle for many bacteria, including recent studies on L. pneumophila. The independent experiments used at biofilm formation by the two L. pneumophila strains, we analyzed (i) the number of days it took for the establishment biofilms, as an indicator of the speed of biofilm formation; and (ii) growth phase at which the microorganismes were observed attachment from the walls, as an indicator of the adherence stability. Interestingly, the ability of L. pneumophila strain reached a maximum level after incubation for 6 days. This is in total accordance with Pécastaings et al[26] in where it was demonstrated that able to adherent and biofilm formation in 6 days. In contrast, the adherence cells greatly increased in the first 6 days and then decreased in the 9 to 12 last days of the experiments. This explain that L. pneumophila is a fastidious and facultative intracellular bacterium with specific growth needs, hence it is assumed that monospecies proliferation (planktonically or in biofilm) in oligotrophic conditions is limited[26]. Furthermore, we have shown that adhesion is highest for late stationary phase cells. This is expected, as L. pneumophila shows increased expression of the flagellum and of type IV pili, both of which have been described in other bacteria to be involved in adhesion[27],[28-29], in stationary phase during low-temperature growth[30-31]. As a result, biofilms are crucial for the economy and to public health because L. pneumophila are associated in a structure that supports their protection and resistance to antimicrobial agents and constitute a threat of infection for the immunocompromised and sensitive.The formation of biofilm by L. pneumophila is affected with various factors (e.g. types of surface materials, water temperature…).However the influence growth temperatures on biofilm formation by L. pneumophila under defined conditions, we found that L. pneumophila formed biofilms at 20 or 37℃ than at 44℃. Since the temperatures 20℃ and 37℃ are often encountered in man-made aquatic environments, e.g., air-conditioning cooling towers in the summer and hot spring spas, the enhanced ability by L. pneumophila to form biofilms on surfaces would likely increase its chance of association with humans.One of the factors responsible for biofilm formation by L. pneumophila is the surface materials, which are used in water systems, where the colonization of bacteria shows affinity on materials which are not inert chemically. Pipe materials for water supply and distribution can generally be classified into one of two generic types, i.e. metallic or plastic. It is well known that micro-organisms can colonize any surface in contact with water. In our work, three surfaces used for examined the ability of L. pneumophila to adhere and to form biofilm at different temperatures. Our observations showed a capacity for biofilm formation when L. pneumophila was submitted to polyethylene and stainless galvanized at 20 and 37℃. Biofilms at 37℃, however, showed an even and extensive mat of considerably greater cell density with the commonly observed water channel structures. These observations are in accordance with studies of Piao et al[32]. In other observation shows that cell density L. pneumophila sg 2-15 better adhesion on the stainless steel and PE than sg 1 at different temperatures, confirming the affinity character of L. pneumophila sg 2-15 and the importance of this property in these processes. In a previous study described by Momba and Nakala[33] were defined that the characteristics of the material composing this surfaces may greatly influence the densities of bacteria fixed in a distribution system. Studies have pointed out that the roughness of the material used for the distribution of potable water contributes to bacterial attachment[34-35].
5. Conclusions
- The management of water quality in distribution systems is a major technological challenge did to human consumption. Vigilance for any contamination and microbial degradation must be maintained. Biofilm is important for the survival and multiplication of L. pneumophila in systems distribution water. Interestingly, the growth of other environmental organisms stimulates the growth of L. pneumophila in aquatic environments. An infectious dose level for L. pneumophila has not been established, however low numbers of this bacterium possess risk for public health. The study demonstrates the distribution of L. pneumophila introduced between growth temperature and biofilm formed on different materials in surfaces system. On the basis of this and other similar studies, we conclude that L. pneumophila was not capable to develop at temperature above 44°C and with use the construction of drinking water distribution systems the copper and other materials similar of copper that did not encourage multiplication of L. pneumophila by supporting bacterial populations in the biofilm which aid the growth of the pathogen. This is particularly so in hospitals, in hotels and in big building domestic where large numbers of susceptible immunosuppressed are exposed to water which may be at the optimum temperature the growth of the pathogen. These results encourage further researches focusing on the capability of L. pneumophila to adhere, detach and form biofilm on surfaces of systems distribution hot water in morocco depending the efficacy classical and alternative sanitizers to reduce the number of cells in the biofilm matrix.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML