-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2026; 14(1): 33-38
doi:10.5923/j.ijge.20261401.06
Received: Dec. 17, 2025; Accepted: Jan. 20, 2026; Published: Feb. 5, 2026

Impact of Hyaluronan-Enriched Transfer Medium on Pregnancy and Live Birth Rates in Euploid Frozen Embryo Transfers Performed in Dry Incubator Conditions
Khalilova Kamolakhon Oybek qizi1, Yunik Tatyana Pavlovna2, Boboyev Sayfulla Gafurovich3
1Ph.D Student, Department of Biology and Ecology, National University of Uzbekistan, Tashkent, Uzbekistan
2Head of Embryology, Israeli Medical Center Reproductive Medicine and Family Health, Tashkent, Uzbekistan
3DSc., Prof., Department of Biology and Ecology, National University of Uzbekistan, Tashkent State Agrarian University, Tashkent, Uzbekistan
Correspondence to: Khalilova Kamolakhon Oybek qizi, Ph.D Student, Department of Biology and Ecology, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2026 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigated the effect of a hyaluronan-enriched embryo transfer medium on reproductive outcomes in euploid frozen embryo transfer (FET) cycles performed under standardized laboratory and clinical conditions. A total of 510 single euploid blastocyst transfers were included. All embryos underwent trophectoderm biopsy followed by NGS-based preimplantation genetic testing for aneuploidy (PGT-A); only embryos without detected chromosomal abnormalities were selected, while mosaic, aneuploid, and inconclusive embryos were excluded from analysis. After warming, embryos were transferred either in a standard transfer medium (control group) or following a 3-hour incubation in a transfer medium enriched with high-molecular-weight hyaluronan. All embryo transfers were performed under ultrasound guidance using standardized protocols. Clinical pregnancy, ongoing pregnancy, and live birth rates were used as primary outcome measures and defined according to international ART reporting standards. The use of a hyaluronan-enriched transfer medium was associated with consistently improved reproductive outcomes. The clinical pregnancy rate was 67.3% in the hyaluronan group compared with 57.4% in the control group, while ongoing pregnancy rates were 56.3% versus 46.9%, respectively. Live birth rates were also higher in the hyaluronan group (57.1%) compared with the control group (46.1%). These findings demonstrate that exposure of euploid blastocysts to a hyaluronan-enriched transfer medium prior to embryo transfer is associated with improved implantation continuity and live birth outcomes. The results support the use of hyaluronan-containing media as a biologically rational strategy to optimize embryo–endometrium interaction and enhance the effectiveness of euploid FET cycles.
Keywords: Hyaluronan-enriched transfer medium, Euploid blastocyst, Frozen embryo transfer, Preimplantation genetic testing, Live birth rate
Cite this paper: Khalilova Kamolakhon Oybek qizi, Yunik Tatyana Pavlovna, Boboyev Sayfulla Gafurovich, Impact of Hyaluronan-Enriched Transfer Medium on Pregnancy and Live Birth Rates in Euploid Frozen Embryo Transfers Performed in Dry Incubator Conditions, International Journal of Genetic Engineering, Vol. 14 No. 1, 2026, pp. 33-38. doi: 10.5923/j.ijge.20261401.06.
1. Introduction
- Implantation failure remains one of the major causes of IVF cycle inefficiency, even when high-quality blastocysts and optimized laboratory protocols are used. Although technological advancements such as improved culture systems, vitrification, and preimplantation genetic testing have significantly enhanced embryo selection, achieving reliable implantation continues to be a clinical challenge [1].Hyaluronan (HA), a key component of the extracellular matrix, plays an essential role in cell adhesion, hydration, and molecular signaling during early implantation. Through its interaction with receptors such as CD44, HA supports trophoblast adhesion and embryo–endometrium communication, providing a strong biological rationale for its use in embryo transfer media [2].The most comprehensive evaluation of HA use in assisted reproduction has been presented in the Cochrane Review by Heymann et al., which analyzed 26 randomized controlled trials involving 6,704 women and demonstrated an absolute increase in live birth rates of approximately 4–11% when high-concentration HA transfer media were used[3]. However, these studies were conducted under conditions that differ substantially from current IVF practice. Specifically:• most trials used humidified incubators, which are subject to greater pH, gas, and temperature fluctuations compared to modern dry incubators [4,5] [5-7].• embryos were not chromosomally screened, making it impossible to isolate the effect of HA from embryo aneuploidy [8,9] [9,10].iven that chromosomal competence is now recognized as the strongest determinant of implantation success, and that IVF laboratory environments have become increasingly standardized and tightly controlled, the true contribution of HA in euploid frozen embryo transfer (FET) cycles remains insufficiently defined. To date, no large-scale study has specifically evaluated the effect of HA within the context of dry-incubator culture systems, which minimize environmental variability and may enhance embryo responsiveness to biologically active transfer media [11].To address this gap, the present study analyzes 510 euploid FET cycles performed under standardized dry-incubator conditions, uniform vitrification–warming protocols, and consistent transfer techniques [12]. By controlling for embryo chromosomal status and laboratory variability, this investigation aims to provide a more precise assessment of the clinical value of hyaluronan-enriched transfer media in a modern, high-precision IVF setting.
2. Materials and Methods
- This controlled clinical study included 510 single euploid frozen embryo transfer (FET) cycles performed in a high-volume assisted reproductive technology (ART) laboratory. All clinical and embryological procedures were conducted according to standardized institutional protocols. Patients were allocated into two groups based on the transfer medium used, while all other laboratory and clinical conditions were kept identical.• Control Group (n = 256): embryos transferred in a standard transfer medium without hyaluronan.• Hyaluronan Group (n = 254): embryos transferred after a 3-hour incubation in a hyaluronan-enriched transfer medium.Ovarian Stimulation, Oocyte Retrieval, and FertilizationControlled ovarian stimulation was performed using recombinant follicle-stimulating hormone (FSH) and/or human menopausal gonadotropin (hMG) in combination with either GnRH antagonist or agonist protocols to prevent premature ovulation. Final oocyte maturation was triggered using human chorionic gonadotropin (hCG) or a GnRH agonist based on individual ovarian hyperstimulation syndrome (OHSS) risk. Oocyte retrieval was performed 34–36 hours after trigger administration. Following cumulus cell removal, only mature metaphase II (MII) oocytes were selected for insemination. To standardize fertilization outcomes and minimize variability, intracytoplasmic sperm injection (ICSI) was performed in all cycles.Embryo Culture and Mechanical Assisted HatchingNormally fertilized two-pronuclear (2PN) embryos were cultured in sequential media under dry incubator conditions (37°C, 6% CO₂, 5% O₂) with oil overlay to maintain environmental stability. On Day 4 of development, embryos underwent mechanical assisted hatching using a microneedle to create a controlled opening in the zona pellucida. This approach facilitated trophectoderm herniation by Day 5–6 and improved access during biopsy, while avoiding the potential thermal stress associated with laser-assisted techniques (Figure 1).
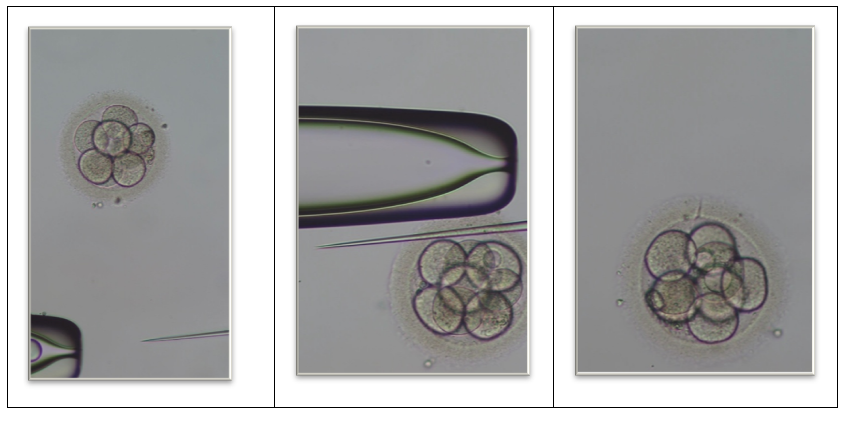 | Figure 1. Mechanical assisted hatching of human embryos |
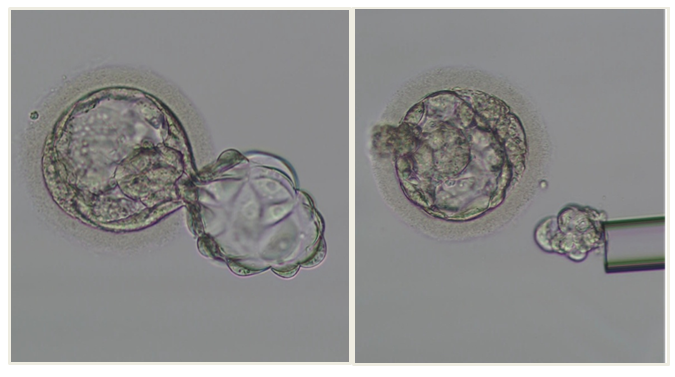 | Figure 2. Trophectoderm biopsy using the flicking technique |
3. Results
- A total of 510 single frozen embryo transfer (FET) cycles were included in the analysis. All embryos underwent NGS-based PGT-A prior to vitrification.PGT-A outcomesPGT-A analysis demonstrated that 68.4% of biopsied blastocysts were classified as euploid and selected for vitrification and subsequent transfer. The remaining 31.6% of embryos exhibited chromosomal abnormalities and were excluded from clinical outcome analysis (Figure 1).Among the abnormal embryos, chromosomal alterations were represented by aneuploid and mosaic abnormal embryos, while samples with inconclusive results were not included in further evaluation. Thus, the study cohort used for transfer analysis consisted exclusively of genetically normal (euploid) embryos, allowing elimination of chromosomal status as a confounding factor.Overall chromosomal assessment showed that 68.4% of biopsied blastocysts were classified as euploid, while 31.6% exhibited chromosomal abnormalities. Only euploid embryos were selected for vitrification and subsequent transfer.Clinical outcomesComparison of reproductive outcomes between study groups demonstrated consistently higher success rates in the hyaluronan group compared with the control group (Figure 3, 4).
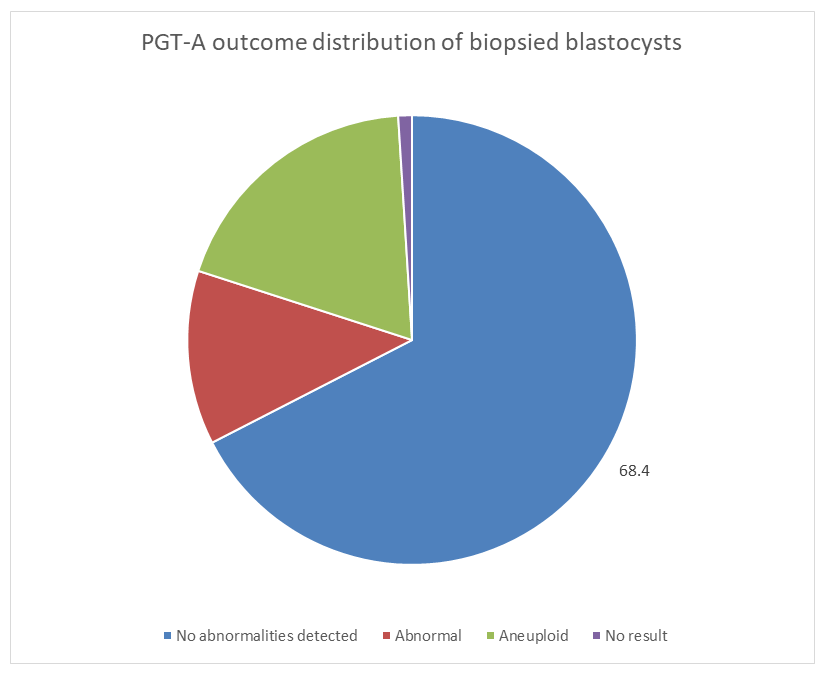 | Figure 3. Distribution of PGT-A results in the study cohort |
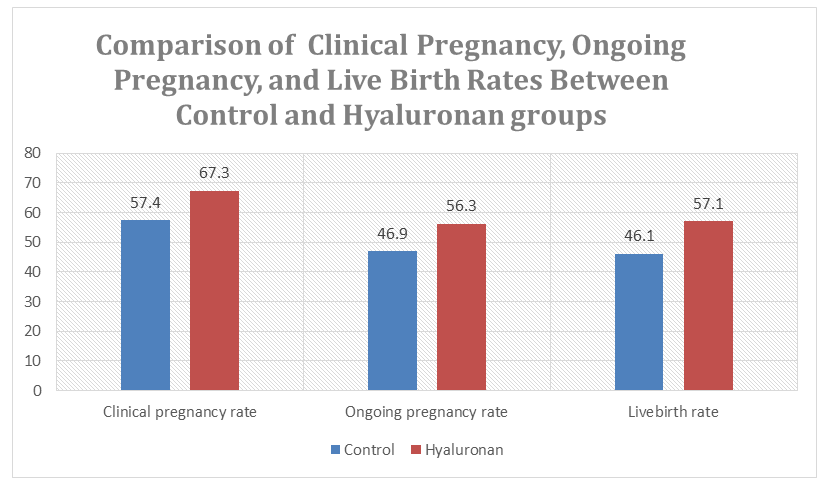 | Figure 4. Clinical pregnancy, ongoing pregnancy, and live birth rates in control vs. hyaluronan groups |
4. Discussion
- The present study demonstrates that incubation of euploid blastocysts in a hyaluronan-enriched transfer medium is associated with improved reproductive outcomes in frozen embryo transfer cycles. By restricting clinical analysis exclusively to embryos with confirmed euploid chromosomal status, the study minimizes genetic confounding and allows a more accurate assessment of the biological contribution of hyaluronan to implantation success.The PGT-A distribution observed in this cohort, with 68.4% of blastocysts classified as euploid and 31.6% exhibiting chromosomal abnormalities, highlights the substantial prevalence of genetic alterations even at the blastocyst stage. This finding reinforces the critical role of chromosomal screening in contemporary IVF practice and underscores the importance of controlling embryo genetic competence when evaluating adjunctive laboratory interventions.The consistently higher rates of clinical pregnancy, ongoing pregnancy, and live birth observed in the hyaluronan group suggest that hyaluronan exerts its primary effect at the level of embryo–endometrium interaction rather than through embryo selection. Hyaluronan is known to facilitate trophoblast adhesion and signaling through interactions with CD44 and other extracellular matrix components, thereby supporting stable implantation and early placental development.Compared with earlier studies and meta-analyses, including the Cochrane Review, the magnitude of improvement observed in this study appears enhanced. This difference may be attributed to the highly standardized laboratory environment employed, including dry-incubator culture, mechanical assisted hatching, flicking-technique trophectoderm biopsy, Cryotop vitrification, and uniform warming protocols. These conditions likely reduce environmental stress and laboratory-induced variability, allowing the biological effects of hyaluronan to be more consistently expressed.Overall, the findings suggest that the effectiveness of hyaluronan-containing transfer media may be maximized in high-precision IVF settings where embryo viability is carefully preserved at every stage of laboratory manipulation.
5. Conclusions
- The outcomes of the present study provide strong and coherent evidence that the use of hyaluronan-enriched transfer medium significantly enhances reproductive success in euploid frozen embryo transfer cycles. Hyaluronan supplementation resulted in higher clinical pregnancy, ongoing pregnancy, and live birth rates compared with standard transfer conditions, with improvements exceeding those reported in international trials and meta-analyses, including the Cochrane Review.Several factors unique to this program likely amplified the observed benefit.First, the exclusive use of euploid embryos removed genetic variability and allowed the true biological effect of hyaluronan to be isolated.Second, the implementation of dry-incubator technology minimized environmental fluctuations in gas composition, temperature, pH, and osmolarity, thereby preserving embryo stability and increasing responsiveness to HA-mediated molecular interactions.Third, a rigorously standardized embryology workflow including mechanical assisted hatching, flicking-method TE biopsy, Cryotop vitrification, and controlled warming ensured optimal embryo viability at all stages.Finally, hyaluronan itself supports a range of critical reproductive mechanisms: enhanced trophectoderm adhesion, improved extracellular matrix structure, modulation of early immune tolerance, and facilitation of trophoblast invasion. These mechanisms collectively contribute to the improved implantation performance and pregnancy continuity observed in this study.Taken together, these findings indicate that hyaluronan is more than a viscosity additive it is a biologically active, clinically meaningful adjunct capable of improving the entire reproductive trajectory from implantation to live birth. The marked increase in live birth rate, our most definitive endpoint, further confirms the value of incorporating hyaluronan-enriched medium into contemporary euploid FET protocols.In conclusion, hyaluronan-enriched transfer medium represents an evidence-based, high-impact enhancement to modern assisted reproduction practices. Its systematic integration into FET programs particularly those utilizing dry incubators and unified embryology standards has the potential to elevate implantation efficiency, reduce early pregnancy loss, and significantly improve overall IVF success rates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML