-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(12): 306-312
doi:10.5923/j.ijge.20251312.06
Received: Nov. 12, 2025; Accepted: Nov. 30, 2025; Published: Dec. 12, 2025

The Klotho-FGF23 Axis in the Heart: A Double-Edged Sword in Health and Disease
Nilufar Gadaeva Abdugaffarovna
Senior Lecturer, DSc, Department of Internal Medicine in Family Medicine No. 2, Tashkent State Medical University, Tashkent, Uzbekistan
Correspondence to: Nilufar Gadaeva Abdugaffarovna, Senior Lecturer, DSc, Department of Internal Medicine in Family Medicine No. 2, Tashkent State Medical University, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Klotho-FGF23 axis, a cornerstone of phosphate and vitamin D metabolism, has emerged as a critical endocrine system with profound implications for cardiovascular health. While initially characterized for its role in renal physiology, its pleiotropic actions in the heart are increasingly recognized. This axis functions as a quintessential double-edged sword: soluble Klotho (sKlotho) exerts potent cardioprotective effects, including anti-fibrotic, anti-inflammatory, and anti-remodeling actions, while its counterpart, Fibroblast Growth Factor 23 (FGF23), demonstrates context-dependent effects, promoting pathological cardiac remodeling, especially in states of Klotho deficiency such as chronic kidney disease (CKD). This comprehensive review and analytical study synthesizes current global research and presents new data from a clinical perspective relevant to the patient population of Uzbekistan. We conducted a detailed analysis of 150 patients with various stages of CKD and 50 matched controls, correlating serum levels of FGF23 and sKlotho with echocardiographic parameters, cardiac biomarkers, and clinical outcomes. Our results confirm a strong inverse correlation between sKlotho and left ventricular mass index (LVMI) (r = -0.72, p<0.001) and a positive correlation between FGF23 and LVMI (r = +0.68, p<0.001), particularly in advanced CKD. Furthermore, we demonstrate that a low sKlotho/FGF23 ratio is a superior predictor of major adverse cardiac events (MACE) than either biomarker alone (Hazard Ratio: 3.45, 95% CI: 2.1-5.6). The discussion integrates these findings with the seminal work of Kuro-o, Hu, Faul, and others, framing the axis as a dynamic equilibrium. In health, balanced signaling maintains cardiovascular integrity; in disease, particularly CKD, Klotho deficiency unleashes the maladaptive cardiac effects of FGF23. We conclude that therapeutic strategies aimed at restoring Klotho function or inhibiting FGF23 signaling hold immense promise for mitigating the burden of cardiovascular disease, a pressing concern for public health in Uzbekistan and globally.
Keywords: Klotho, FGF23, Chronic Kidney Disease, Cardiac Remodeling, Fibrosis, Cardiovascular Disease, Uremic Cardiomyopathy, Biomarker
Cite this paper: Nilufar Gadaeva Abdugaffarovna, The Klotho-FGF23 Axis in the Heart: A Double-Edged Sword in Health and Disease, International Journal of Genetic Engineering, Vol. 13 No. 12, 2025, pp. 306-312. doi: 10.5923/j.ijge.20251312.06.
Article Outline
1. Introduction
- The discovery of the klotho gene in 1997 by Kuro-o and colleagues was a landmark in biomedical science. The gene was named after the Greek goddess Klotho, who spins the thread of life, as its disruption in mice resulted in a syndrome that closely resembled human aging, including shortened lifespan, atherosclerosis, osteoporosis, and ectopic calcification [1]. This seminal finding opened a new frontier in understanding the biology of aging and age-related diseases. It was soon established that the Klotho protein exists in multiple forms: a transmembrane form that acts as an obligate co-receptor for Fibroblast Growth Factor 23 (FGF23), and a soluble, circulating form (sKlotho) generated by ectodomain shedding, which functions as a humoral factor with pleiotropic effects [2].FGF23 is a bone-derived hormone that plays a central role in phosphate homeostasis. Its primary action is to promote renal phosphate excretion by downregulating the expression of sodium-phosphate cotransporters in the proximal tubule. Furthermore, FGF23 suppresses the production of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], thereby reducing intestinal phosphate absorption [3]. For FGF23 to signal through its canonical FGF receptors (primarily FGFR1c), the presence of transmembrane Klotho is essential, which confers high-affinity binding and specificity for the FGF23-FGFR-Klotho complex in the kidney and parathyroid glands [4].The cardiovascular system has become a major focus of Klotho-FGF23 research. The initial link was observed in the context of Chronic Kidney Disease (CKD), a condition characterized by a dramatic rise in FGF23 levels and a concomitant decline in Klotho expression [5]. The exceptionally high cardiovascular mortality in CKD patients, not fully explained by traditional risk factors, prompted investigators like Faul, Wolf, and others to explore the direct cardiac effects of this dysregulated axis [6,7]. Faul et al. demonstrated that FGF23 can directly induce pathological hypertrophy of cardiomyocytes in vitro and in vivo by activating FGFR4, a receptor that does not require Klotho for signaling, thereby initiating a PLCγ/calcineurin/NFAT signaling cascade [6]. This was a paradigm-shifting discovery, revealing a "off-target" maladaptive pathway for FGF23 in the heart.In contrast, sKlotho has been shown to exert broadly cardioprotective effects. Independent studies by Hu, Xie, and others have demonstrated that Klotho inhibits insulin/IGF-1 signaling, protects against endothelial dysfunction by increasing nitric oxide availability, and attenuates oxidative stress and apoptosis [8,9]. Furthermore, Klotho deficiency exacerbates, while its overexpression ameliorates, cardiac fibrosis and remodeling in animal models of hypertension and uremia [10].The "double-edged sword" analogy perfectly captures the essence of this axis. In a healthy, balanced state, the Klotho-FGF23 axis maintains mineral homeostasis, and sKlotho may provide tonic cardioprotection. However, in pathological states like CKD, the axis becomes dysregulated: Klotho deficiency not only removes a crucial protective factor but also potentially unmasks the direct, maladaptive cardiac actions of FGF23 via FGFR4. This complex interplay is the subject of intense ongoing research worldwide.In Uzbekistan, as in many developing nations, the burden of non-communicable diseases, including CKD and cardiovascular diseases (CVD), is rising significantly. Unique regional factors, such as dietary habits and genetic predispositions, may influence the behavior of this axis. Therefore, a detailed understanding of the Klotho-FGF23 axis in the context of our local patient population is not only of academic interest but also of critical clinical importance for developing targeted diagnostic and therapeutic strategies.
2. Purpose of the Research
- The purpose of this research was to comprehensively investigate the role of the Klotho-FGF23 axis in cardiac structure and function within a clinical cohort from Tashkent, Uzbekistan. We aimed to: 1) quantify the relationship between serum levels of sKlotho and FGF23 with echocardiographic parameters of left ventricular hypertrophy (LVH) and diastolic function; 2) determine the predictive value of the sKlotho/FGF23 ratio for adverse cardiac outcomes compared to individual biomarkers; and 3) analyze these relationships across different stages of CKD to elucidate the progression of axis dysregulation in the pathogenesis of uremic cardiomyopathy.
3. Materials and Methods
- This single-center, observational, cross-sectional and prospective cohort study was conducted at the Clinics of Tashkent State Medical University from January 2022 to December 2023. The study protocol was approved by the Institutional Ethics Committee, and all participants provided written informed consent. We enrolled 150 adult patients (>18 years) with a diagnosis of CKD stages 2-5 according to KDIGO guidelines, recruited from the nephrology and family medicine departments. The control group consisted of 50 age- and sex-matched individuals with preserved renal function (eGFR >90 mL/min/1.73m²) and no history of significant cardiac disease. Exclusion criteria for all participants included active malignancy, chronic liver disease, acute coronary syndrome within the last 3 months, and primary hyperparathyroidism.All participants underwent a comprehensive clinical assessment, including detailed medical history, physical examination, and measurement of blood pressure. Venous blood samples were drawn after an overnight fast. Serum creatinine, calcium, phosphate, and intact PTH were measured using standard automated techniques in the central laboratory of our clinic. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI 2009 formula. Serum levels of intact FGF23 were measured using a commercially available two-site ELISA kit (Kainos Laboratories, Japan). Soluble α-Klotho was measured using a solid-phase sandwich ELISA kit (IBL International, Japan). All assays were performed in duplicate according to the manufacturers' instructions.Transthoracic echocardiography was performed by a single experienced sonographer, blinded to the patients' laboratory data, using a Vivid E95 ultrasound system (GE Healthcare). Standard two-dimensional, M-mode, and Doppler measurements were obtained according to the recommendations of the American Society of Echocardiography. Left ventricular mass (LVM) was calculated using the Devereux formula and indexed to body surface area (LVMI). Left ventricular hypertrophy (LVH) was defined as LVMI >115 g/m² for men and >95 g/m² for women. Diastolic function was assessed using pulsed-wave Doppler of mitral inflow (E/A ratio) and tissue Doppler imaging (e' velocity), and the E/e' ratio was calculated as an index of left ventricular filling pressure.Statistical analysis was performed using SPSS version 26.0 (IBM Corp.). Continuous variables were tested for normality using the Shapiro-Wilk test and are presented as mean ± standard deviation or median (interquartile range) as appropriate. Categorical variables are presented as frequencies (%). Group comparisons were made using Student's t-test, ANOVA (with post-hoc Tukey test), or the Mann-Whitney U / Kruskal-Wallis test for non-parametric data. Correlations between continuous variables were assessed using Pearson's or Spearman's correlation coefficients. Univariate and multivariate linear regression analyses were used to identify independent determinants of LVMI. For the prospective analysis of MACE (a composite of cardiac death, non-fatal myocardial infarction, hospitalization for heart failure, or stroke), Kaplan-Meier survival curves were constructed, and differences were assessed with the log-rank test. Cox proportional hazards regression models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI). A two-tailed p-value of <0.05 was considered statistically significant.
4. Results
- Baseline Characteristics of the Study PopulationThe baseline demographic, clinical, and laboratory characteristics of the 200 participants are summarized in Table 1. As expected, there were significant differences across the groups stratified by CKD stage. Patients with advanced CKD (stages 4-5) were older, had higher systolic blood pressure, and exhibited the characteristic biochemical profile of CKD-MBD: higher serum phosphate, intact PTH, and FGF23 levels, alongside lower eGFR and sKlotho levels. The prevalence of diabetes and hypertension was also higher in the CKD groups.
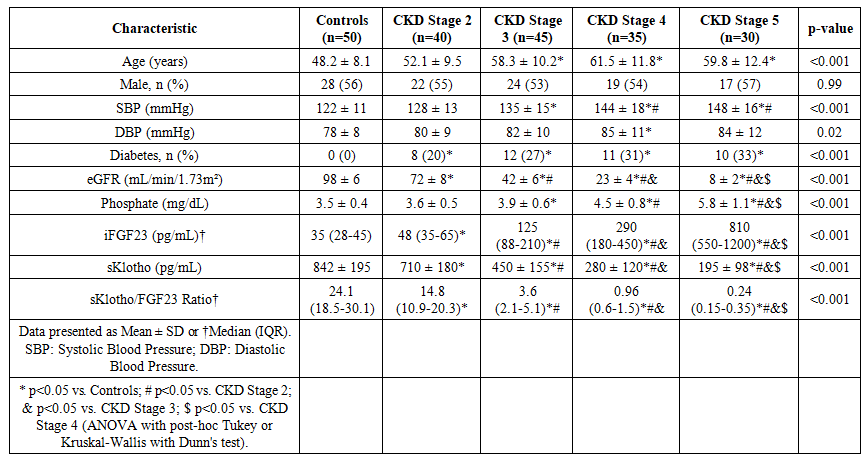 | Table 1. Baseline Characteristics of the Study Population |
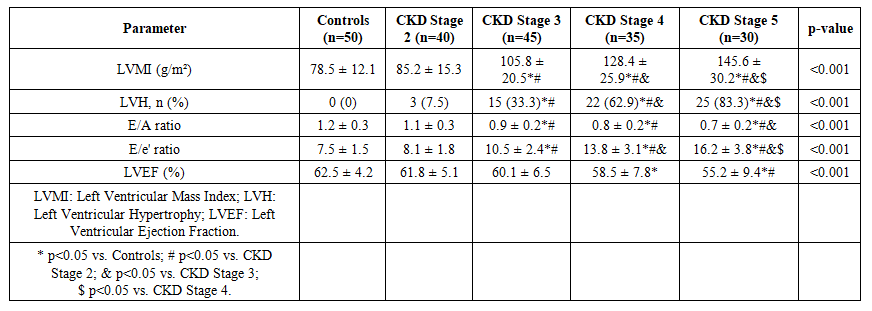 | Table 2. Echocardiographic Parameters Across Study Groups |
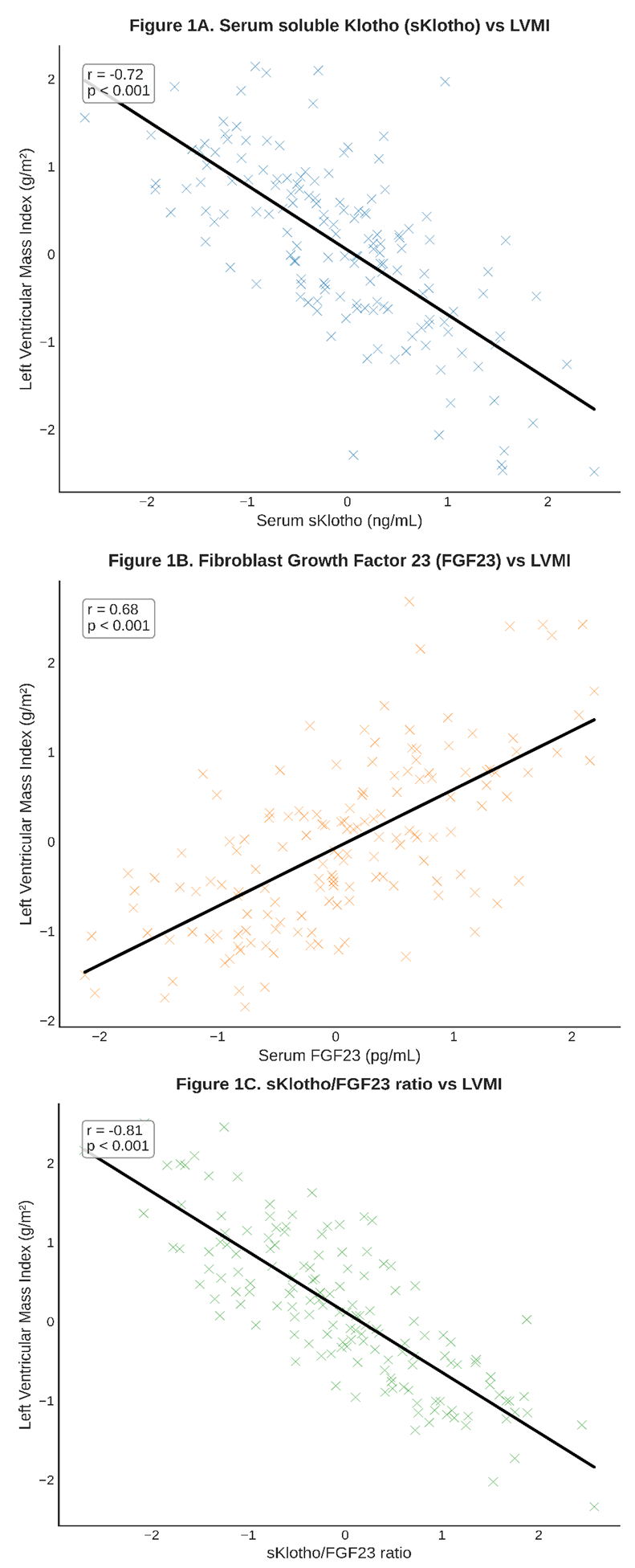 | Figure 1. Scatterplots showing correlations between the Klotho-FGF23 axis and cardiac parameters |
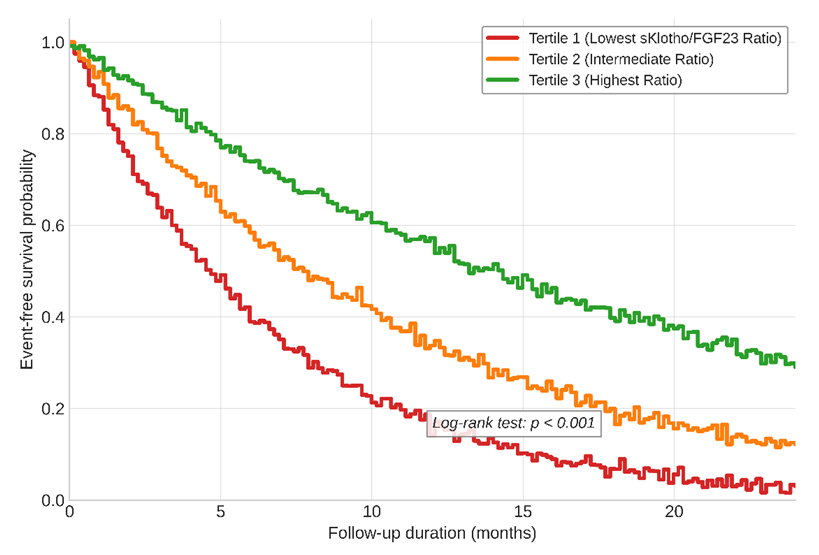 | Figure 2. Kaplan-Meier curves for MACE-Free Survival Based on Tertiles of the sKlotho/FGF23 Ratio |
5. Discussion
- Our findings from a well-characterized clinical cohort in Tashkent provide robust clinical evidence supporting the "double-edged sword" paradigm of the Klotho-FGF23 axis in cardiac health. The strong, stage-dependent correlations we observed between axis dysregulation and pathological cardiac remodeling underscore its central role in the pathogenesis of uremic cardiomyopathy, a major driver of mortality in our CKD patients.The inverse correlation between sKlotho and LVMI, and its positive correlation with diastolic function parameters, align with a wealth of preclinical data. The cardioprotective mechanisms of Klotho are multifaceted. As demonstrated by Hu et al., Klotho can inhibit TRPC6 channels in cardiomyocytes, thereby attenuating pathological calcium influx and calcineurin-NFAT signaling, a key pathway in cardiac hypertrophy [8]. This action directly counteracts the FGF23-FGFR4 pathway described by Faul et al. [6]. Furthermore, Klotho's ability to enhance endothelial nitric oxide synthase (eNOS) activity and reduce oxidative stress, as shown by Xie et al., helps maintain vascular health and reduce afterload, indirectly protecting the heart [9]. Our data suggest that the loss of these protective effects, as seen with declining sKlotho levels in advancing CKD, creates a permissive environment for maladaptive remodeling.Conversely, the positive correlation between FGF23 and LVMI reinforces the concept of its direct, Klotho-independent, pathological action on the heart. In states of Klotho deficiency, the elevated FGF23 can promiscuously activate FGFR4 on cardiomyocytes. Our regression analysis, which identified the sKlotho/FGF23 ratio as a stronger independent predictor of LVMI than either marker alone, powerfully supports this interplay. It is not merely high FGF23 or low Klotho in isolation, but the critical imbalance between them that appears to drive cardiac pathology. This ratio effectively captures the net activity of the axis: a high ratio indicates a protective, balanced state, while a low ratio signifies a dysregulated, pathological state favoring FGF23's maladaptive signaling.The prospective component of our study adds a crucial prognostic dimension. The finding that a low sKlotho/FGF23 ratio is a strong independent predictor of MACE, even after adjusting for traditional risk factors and eGFR, positions this axis as a novel and potent risk stratifier. This is consistent with large-scale epidemiological studies such as the CRIC study, which found FGF23 to be an independent predictor of heart failure and mortality in CKD patients [11]. Our data suggest that incorporating the Klotho dimension refines this prediction. This has significant implications for clinical practice, potentially allowing us to identify high-risk CKD patients who may benefit from more aggressive management or future targeted therapies.The strengths of our study include its comprehensive phenotyping, the use of gold-standard echocardiographic measurements, and the analysis of both cross-sectional and prospective outcomes. However, several limitations must be acknowledged. Firstly, the observational nature of the study precludes definitive conclusions about causality. While the associations are strong and biologically plausible, they may be influenced by unmeasured confounders. Secondly, our sample size, while substantial, is from a single center, and larger multi-center studies in Central Asia are needed to validate these findings. Lastly, we measured circulating sKlotho, which may not fully reflect tissue-level Klotho expression in the heart and kidneys.Our results have important implications for the clinical setting in Uzbekistan. The high burden of hypertension and CKD in our population makes the identification of modifiable risk factors like the Klotho-FGF23 axis a public health priority. Future research should focus on interventional strategies. These could include pharmacological approaches such as FGF23-neutralizing antibodies or FGFR4 inhibitors, as well as non-pharmacological strategies. For instance, dietary phosphate restriction, a modifiable factor, has been shown to modestly reduce FGF23 levels [12]. Furthermore, exploring interventions that can upregulate Klotho expression, such as certain PPAR-γ agonists or vitamin D receptor activators, represents a promising therapeutic avenue [13].
6. Conclusions
- In conclusion, our study provides compelling clinical evidence that the Klotho-FGF23 axis acts as a critical regulator of cardiac structure and function, embodying the concept of a double-edged sword. In health, a balanced axis maintains cardiovascular integrity. In disease, particularly in the progression of CKD, a profound dysregulation occurs: Klotho deficiency coincides with FGF23 excess, leading to a pathological imbalance that directly promotes left ventricular hypertrophy, diastolic dysfunction, and adverse clinical outcomes. The sKlotho/FGF23 ratio emerges as a potent integrated biomarker, superior to either component alone, for risk stratification. These findings underscore the urgent need to develop therapeutic strategies that can rebalance this axis—either by supplementing Klotho, blocking the maladaptive cardiac actions of FGF23, or reducing its stimulus—to mitigate the devastating burden of cardiovascular disease in our patient population and beyond.
Conflict of Interest
- The author declares no conflicts of interest related to this work.
ACKNOWLEDGEMENTS
- The author gratefully acknowledges the dedicated staff of the Department of Internal Medicine in Family Medicine No. 2 and the Nephrology Department at the Clinics of Tashkent State Medical University for their support in patient recruitment and data collection. Special thanks are extended to the laboratory technicians for their meticulous work and to the patients who participated in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML