-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(12): 279-285
doi:10.5923/j.ijge.20251312.01
Received: Oct. 27, 2025; Accepted: Nov. 23, 2025; Published: Dec. 9, 2025

Rust Fungi of the Surkhan State Nature Reserve
Mallayev Muslim Khushnazar ugli1, Mustafayev Ilyor Muradullayevich2, Turaboev Mirzarahmat Bakhtiyor ugli3, Atoyev Kayhon Uktamovich4
1PhD Student at the Denov Institute of Entrepreneurship and Pedagogy, Surkhandarya, Uzbekistan
2PhD of Biological, Senior Researcher, F.N. Rusanov Tashkent Botanical Garden, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
3Junior Researcher, F.N. Rusanov Tashkent Botanical Garden, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
4Lecturer, Department of Forestry, Medicinal Plants, and Landscape Gardening, Termiz State University of Engineering and Agro-technologies, Surkhandarya, Uzbekistan
Correspondence to: Mallayev Muslim Khushnazar ugli, PhD Student at the Denov Institute of Entrepreneurship and Pedagogy, Surkhandarya, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

During 2020–2025, extensive field investigations were conducted in the Surkhan State Reserve, during which rust fungi (Pucciniales) specimens were collected and subjected to comprehensive mycological analyses. The results revealed a total of 59 rust species belonging to 4 families and 6 genera. The most species-rich genera were Puccinia (33 species), Uromyces (12 species), and Phragmidium (8 species), while Gymnosporangium, Aecidium, and Melampsora were represented by 2–3 species each. Notably, Puccinia hsinganensis (on Fritillaria bucharica Regel.) was recorded for the first time in the mycobiota of Uzbekistan. Rust fungi were found parasitizing 81 plant species representing 50 genera and 24 families. Moreover, seven fungal species were documented for the first time on new host plants within the flora of Uzbekistan, and three species were observed parasitizing plants listed in the Red Data Book of the Republic of Uzbekistan. These findings provide critical insights into the diversity of rust fungi and host–pathogen interactions within the unique ecosystems of southern Uzbekistan.
Keywords: Pucciniales, Micromycete, Uzbekistan, Uromyces, Phragmidium, Puccinia
Cite this paper: Mallayev Muslim Khushnazar ugli, Mustafayev Ilyor Muradullayevich, Turaboev Mirzarahmat Bakhtiyor ugli, Atoyev Kayhon Uktamovich, Rust Fungi of the Surkhan State Nature Reserve, International Journal of Genetic Engineering, Vol. 13 No. 12, 2025, pp. 279-285. doi: 10.5923/j.ijge.20251312.01.
1. Introduction
- Rust fungi (Pucciniales) represent the largest and biologically most significant group of obligate biotrophic micromycetes belonging to the division Mycota. According to modern phylogenetic classifications, they are united within a single order — Pucciniales, which comprises 14 families, 168 genera, and more than 7,000 species [15]. These obligate parasitic fungi possess a complex ontogenetic life cycle that includes five distinct spore stages: basidiospores, spermatia, aeciospores, urediniospores, and teliospores [38].These pathogens cause significant economic losses in global agriculture every year. In particular, crops of strategic importance such as wheat, maize, coffee, and soybean are highly susceptible to rust infections, which have a direct negative impact on both yield and product quality. At the same time, some rust fungi species have been successfully used in the biological control of invasive weeds. For example, Phragmidium violaceum [35] and several Puccinia species [32,3] are considered effective biocontrol agents in this regard.Rust fungi are among the most widely studied groups of phytopathogens worldwide, and research in this field has undergone several distinct stages of development in Uzbekistan as well. Studies on rust fungi in Uzbekistan were first initiated by Zaprometov between 1912 and 1926 [36,37], and later continued by Golovin from 1947 to 1950 [9]. During the period from 1950 to 1986, extensive investigations were carried out by N.I. Gaponenko, T.S. Panfilova, T.K. Rotkevich, S.S. Ramazanova, F.G. Akhmedova, and G.T. Baymuratova, which significantly expanded knowledge of rust fungi in the country. Notably, in 1986, Ramazanova and colleagues reported 261 species of rust fungi parasitizing 511 host plant species belonging to 43 families in their work “Flora of Fungi of Uzbekistan” [27].In 1989, Soliyeva defended her doctoral dissertation on the micromycetes of higher plants in the Surkhandarya region. In this work, she reported 352 species of micromycetes belonging to 82 genera and 19 families occurring within the region. Among them, 70 species representing seven genera were identified as rust fungi [29].In recent years, checklists showing the distribution of fungi and their host plants in different regions of Uzbekistan have been provided by [6,7,8,18,20,21,12,13]. In addition, several new species have been documented for the mycobiota of Uzbekistan [22,24].However, until the present study, the mycobiota of the Kugitang mountain range and the Surkhan State Reserve had not been sufficiently investigated. The aim of this study was to compile a comprehensive checklist of rust fungi occurring in the Surkhan State Reserve. This checklist serves as an important source for future mycological monitoring and for updating the national inventory of species diversity within this fungal group.Study areaHerbarium specimens of rust fungi were collected between 2020 and 2025 within the territory of the Surkhan State Reserve, located in southern Uzbekistan. The reserve is situated in the Sherobod District of the Surkhandarya Region, on the eastern slopes of the Kugitang Range, which forms the southwestern part of the Pamir–Alay mountain system. It was established on September 8, 1986, and covers a total area of 23,406 hectares. The altitude of the territory ranges from 850 to 3,137 meters above sea level and is characterized by a complex geomorphological structure (Fig. 1).
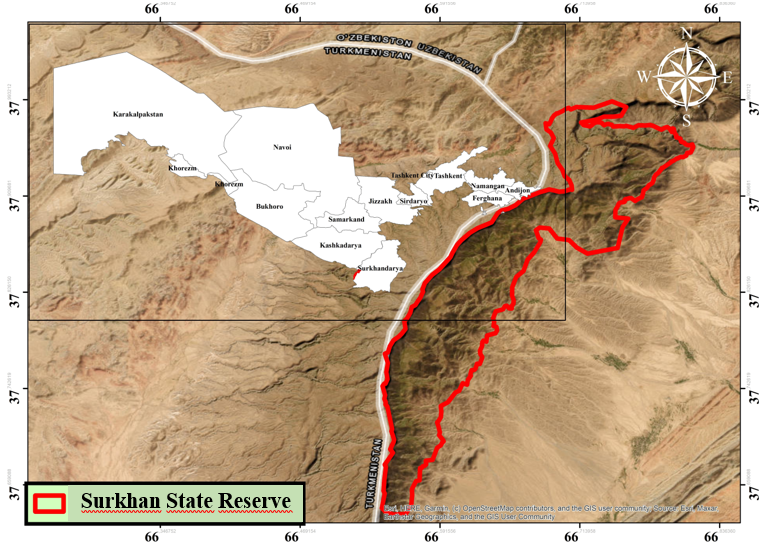 | Figure 1. Map of the Surkhan State Reserve |
2. Material and Methods
- During the course of this research, herbarium specimens of plants infected with rust fungi were collected from various sectors of the Surkhan State Reserve, totaling over 500 samples. The sectors of the reserve are abbreviated as follows: VdB — Vandob sector (37.433275°N, 66.342535°E), ShjB — Sherjon (37.445632°N, 66.362533°E), KtB — Kampirtepa sector (37.474580°N, 66.874894°E), ShqB — Shalqon sector (37.514741°N, 66.380170°E), QoB — Qizilolma sector (37.533045°N, 66.415706°E), XaB — Xo‘jaanqo sector (37.541551°N, 66.444146°E), and XtB — Xatak sector (37.561775°N, 66.461714°E).The morphology of herbarium specimens was examined using a light microscope, and identification was carried out based on relevant literature [16,14,2,17,33,27]. Host plant species were determined using the “Flora of Uzbekistan” publications [4]. All collected specimens are deposited in the herbarium of the Botanical Institute of the Academy of Sciences of the Republic of Uzbekistan (TASM). Representative specimens of each rust fungus species are indicated in the checklist. The collector’s abbreviation is MM — Muslim Mallayev. The taxonomy and nomenclature of rust fungi follow the international database Index Fungorum [11], while host plant nomenclature is based on data from powo.science.kew.org [26].
3. Results
- During 2020–2025, field studies were conducted by the authors in Surkhan State Reserve, Uzbekistan, focusing on the collection of herbarium specimens of rust fungi (Pucciniales) and their subsequent mycological analysis. The results of these investigations revealed a total of 59 species belonging to 6 genera and 4 families. In terms of species diversity, the genus Puccinia was the most species-rich, comprising 33 species, followed by Uromyces with 12 species and Phragmidium with 8 species. The remaining genera—Gymnosporangium, Aecidium, and Melampsora—were represented by two or three species each.The rust fungi species identified exhibited high taxonomic diversity, with each species being confirmed based on morphological characteristics, microscopic structural features, and host plant specificity. Below is a list of rust fungi species belonging to the order Pucciniales recorded within the territory of Surkhan State Reserve.List of Rust Fungi Identified from the Study AreaMelampsoraceaeMelampsora CastagneM. euphorbiae (Ficinus & C. Schub.) Castagne – on Euphorbia esula subsp. esula , ShqB, 25.V.2024, MM188; 11.V.2025, MM187.M. hissarica Faizieva – on Salix alba L., ShqB, 25.V.2024 MM 418; ShjB, 20.V.2025 MM 427.M. laricis-tremulae Kleb. – on Populus alba L., ShjB, 20.V.2025 MM 431; XtB, 23.IV.2025, MM 487.Phragmidiaceae Phragmidium LinkPh. asiae-mediae L.I. Vassiljeva – on Geum kokanicum Regel & Schmalh., ShqB, 11.V.2025, MM301.Ph. circumvallatum Magnus – on Geum kokanicum Regel & Schmalh., ShqB, 11.V.2025, MM167; 15.VI.2024, MM168.Ph. devastatrix Sorokin – on Rosa kokanica (Regel) Regel ex Juz., ShqB, 11.V.2025, MM036; XtB, 23.IV.2025, MM037; on Rosa ecae Kanitz, XtB, 01.VI.2025, MM039; XtB, 27.IV.2024, MM040; VdB, 02.V.2025, MM041.Ph. kamtschatkae (H.W. Anderson) Arthur & Cummins – on Rosa canina L., XtB, 01.VI.2025, MM032; on Rosa kokanica (Regel) Regel ex Juz., QoB, 04.V.2025, MM35; ShqB, 24.IV.2025, MM38.Ph. mucronatum (Pers.) Link – on Rosa canina L., XtB, 23.IV.2025, MM033.Ph. rosae-lacerantis Dietel – on Rosa ecae Aitch., XtB, 01.VI.2025, MM302.Ph. sanguisorbae (DC.) J. Schröt – on Sanguisorba minor Scop., Shqb, 11.V.2025, MM173; VdB, 02.V.2025, MM175; XaB, 25.IV.2025, MM193.Ph. tuberculatum Jul. Müll. – on Rosa canina L., ShqB, 15.VI.2025, MM010; ShqB, 11.V.2025, MM031; on Rosa ecae Kanitza, ShqB, 23.VIII.2025, MM240.Pucciniaceae Aecidium Pers.Aecidium sp. – on Diarthron vesiculosum (Fisch. & C.A.Mey. ex Kar. & Kir.) C.A.Mey., ShqB, 11.V.2025, MM303.A. thalictri-flavi (DC.) G. Winter – on Thalictrum sp. ShjB, 20.V.2025. MM421.Puccinia Pers.P. absinthii DC. – on Artemisia juncea Kar. & Kir., ShjB, 20.V.2025, MM420; on Artemisia oliveriana J.Gay ex Besser, QoB, 04.V.2025, MM424; XaB, 17.VI.2025, MM422; on Artemisia tenuisecta Nevski, KtB, 14.VI.2025, MM 437.P. aegilopis Maire – on Solenanthus circinnatus Ledeb., KtB, 14.VI.2025, MM298. P. achilleae Cooke – on Handelia trichophylla (Schrenk) Heimerl, ShqB, 25.V.2025, MM499.P. bromina Erikss., Annls Sci. – on Bromus sterilis L., ShjB, 20.V.2025, MM423;P. bulbocastani (A. Cumino) Fuckel – on Elwendia chaerophylloides (Regel & Schmalh.) Pimenov & Kljuykov, XtB, 13.IV.2025, MM084; VDB, 2.V.2025, MM095; on Bunium sp., ShqB, 11.V.2025, MM096.P. calcitrapae DC. – on Centaurea virgata subsp. squarrosa (Boiss.) Gugler, ShqB, 01.VI.2025, MM003; KtB, 14.VI.2025, MM083.P. centaureae Mart. – on Rhaponticum repens (L.) Hidalgo, ShjB, 20.V.2025, MM439.P. cesatii J. Schröt. – on Bothriochloa ischaemum (L.) Keng, VdB, 02.V.2025, MM239;P. cichorii (DC.) Bellynck – on Cichorium intybus L., ShqB, 23.VIII.2025, MM430.P. cnici H. Mart. – on Picnomon acarna (L.) Cass., ShqB, 25.V.2024, MM440.P. conclusa Thüm. – on Cyperus longus L., ShqB, 23.VIII.2025, MM260.P. cousiniae P. Syd. & Syd. – on Cousinia integrifolia Franch., XaB, 25.IV.2025, MM130; Shqb, 24.IV.2025, MM131; on Cousinia glabriseta Kult., XtB, 01.VI.2025, MM132; on Cousinia coronata Franch., ShqB, 15.VI.2025, MM133; XtB, 24.VIII.2025, MM210; on Cousinia sp., QoB, 04.V.2025, MM137; VdB, 02.V.2025, MM138; on Cousinia radians Bunge, QoB, 04.V.2025, MM139; ShqB, 25.V.2024, MM140; on Cousinia resinosa Juz., ShqB, 15.VI.2025, MM149; on Cirsium arvense var. arvense, ShqB, 11.V.2025, MM172.P. cynodontis Lacroix ex Desm. – on Delphinium sp2., QoB, 04.V.2025, MM103.P. eremuri Kom. – on Eremurus kaufmannii Regel, ShqB, 25.V.2024, MM304.P. ferulae-songaricae Tranzschel & Erem – on Ferula kuhistanica Korovin, ShqB, 24.IV.2025, MM014; XaB, 25.IV.2025, MM043; QoB, 04.V.2025, MM044; XaB, 30.IV.2024, MM050.P. fuckelii Körn. – on Jurinea gracilis Iljin, XtB, 01.VI.2025, MM012; XaB, 25.IV.2025, MM097; KtB, 14.VI.2024, MM106; on Jurinea maxima C.Winkl., ShqB, 11.V.2025, MM015.Note: Recorded for the first time on J. maxima (Fig. 2).
 | Figure 2. A – Herbarium specimen of Jurinea maxima C. Winkl. infected with Puccinia fuckelii Körn. B – Microscopic image of Puccinia fuckelii spores |
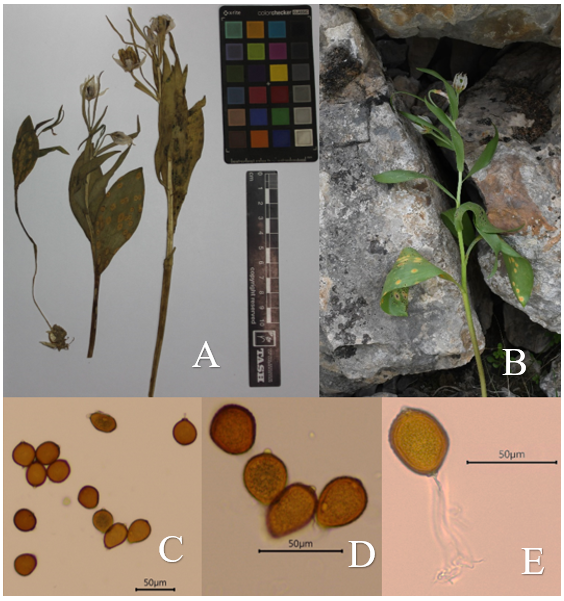 | Figure 3. A – Herbarium specimen of Fritillaria bucharica infected with Puccinia hsinganensis; B – Living Fritillaria bucharica plant; C – Microscopic image of Puccinia hsinganensis spores |
 | Figure 4. A – Herbarium specimen of Acantholimon majewianum Regel infected with Uromyces acantholimonis Syd. & P. Syd.; B, C – Microscopic images of Uromyces acantholimonis spores |
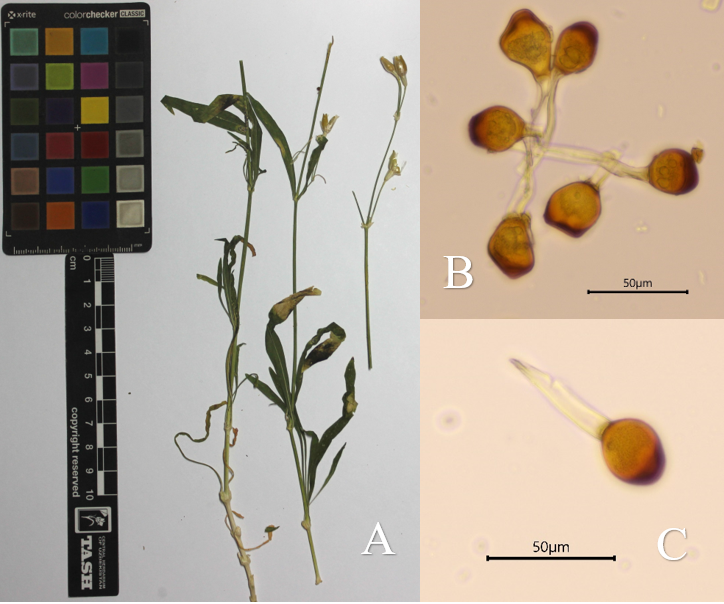 | Figure 5. A – Herbarium specimen of Silene kuschakewiczii Regel & Schmalh. infected with Uromyces verruculosus J. Schröt.; B, C – Microscopic images of Uromyces verruculosus spores |
4. Discussion
- Rust fungi were found parasitizing 81 plant species belonging to 50 genera and 24 families within the reserve. The most affected plant families were Asteraceae (21 species), Rosaceae (10 species), Apiaceae (6 species), Ranunculaceae (6 species), and Fabaceae (5 species). Additionally, seven rust fungi species were recorded for the first time on new host plants in the flora of Uzbekistan, including: Gymnosporangium confusum Plowr. on Pyrus regelii Rehder, Puccinia punctata Link on Galium tricornutum Dandy, P. recondita Roberge ex Desm. on Delphinium sp., P. fuckelii Körn. on Jurinea maxima C. Winkl. (Fig. 2), P. cynodontis Lacroix ex Desm. on Delphinium sp. 2, Uromyces verruculosus J. Schröt. on Silene kuschakewiczii Regel & Schmalh. (Fig. 5), and U. acantholimonis Syd. & P. Syd. on Acantholimon majewianum Regel (Fig. 4).It was also recorded that several rust fungi species parasitize three plant species listed in the Red Data Book of the Republic of Uzbekistan. These include Uromyces hedysari-obscuri (DC.) Carestia & Picc. on Hedysarum magnificum Kudr., Puccinia cousiniae P. Syd. & Syd. on Cousinia glabriseta Kult., and P. fuckelii Körn. on Jurinea gracilis Iljin [28]. Additionally, rust diseases were observed on many medicinal plants within the reserve. These include Ziziphora clinopodioides, Taraxacum officinale, Origanum vulgare subsp. gracile, Ferula kuhistanica, Glycyrrhiza glabra, Thalictrum isopyroides, Mentha longifolia var. asiatica, Malva neglecta, Cichorium intybus, Artemisia oliveriana, Hypericum scabrum, H. perforatum, Rosa canina, among others.It is widely recognized that the diversity of rust fungi exhibits a strong correlation with the richness of the vascular plant flora. The flora of Uzbekistan comprises over 4,500 species of higher plants, among which 261 species of rust fungi, belonging to 43 families, have been recorded on 511 plant species [27] The flora of the Surkhan State Nature Reserve consists of 743 species distributed across 372 genera and 77 families. Within this flora, 59 species of rust fungi have been identified on 81 plant species representing 24 families.It should be emphasized that studies on the diversity of rust fungi have been conducted by several mycologists in various regions of Uzbekistan, including the Angren River basin and the Fergana Valley [25,27,5]. The diversity of rust fungi in the Surkhan State Nature Reserve was comparatively analyzed against the results of these previous investigations (Table 1).
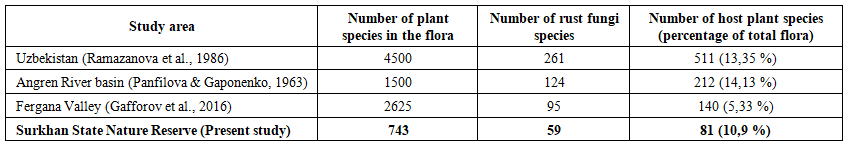 | Table 1. Comparative analysis of rust fungi diversity across different regions of Uzbekistan |
5. Conclusions
- This catalogue provides the first comprehensive list of all rust fungi species recorded within the Surkhan State Nature Reserve since its establishment in 1986. The present work contributes to the development of a more complete synopsis of the rust fungi flora of Uzbekistan and serves as a foundation for further research on the diversity and distribution of mycomycetes across the region.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML